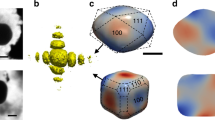
Abstract
Geometry and confinement effects at the nanoscale can result in substantial modifications to a material’s properties with significant consequences in terms of chemical reactivity, biocompatibility and toxicity1,2. Although benefiting applications across a diverse array of environmental and technological settings, the long-term effects of these changes, for example in the reaction of metallic nanoparticles under atmospheric conditions, are not well understood. Here, we use the unprecedented resolution attainable with aberration-corrected scanning transmission electron microscopy3 to study the oxidation of cuboid Fe nanoparticles. Performing strain analysis at the atomic level, we reveal that strain gradients induced in the confined oxide shell by the nanoparticle geometry enhance the transport of diffusing species, ultimately driving oxide domain formation and the shape evolution of the particle. We conjecture that such a strain-gradient-enhanced mass transport mechanism may prove essential for understanding the reaction of nanoparticles with gases in general, and for providing deeper insight into ionic conductivity in strained nanostructures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
11 November 2013
In the version of this Letter originally published online, part of the address of the fifth affiliation was incorrect; it should have read "Frederick Seitz Materials Research Laboratory". This error has now been corrected in all versions of the Letter.
References
Newton, M. C., Leake, S. J., Harder, R. & Robinson, I. K. Three-dimensional imaging of strain in a single ZnO nanorod. Nature Mater. 9, 120–124 (2010).
Gilbert, B., Huang, F., Zhang, H., Waychunas, G. A. & Banfield, J. F. Nanoparticles: Strained and stiff. Science 305, 651–654 (2004).
Kimoto, K. et al. Element-selective imaging of atomic columns in a crystal using STEM and EELS. Nature 450, 702–704 (2007).
Brillet, J. et al. Highly efficient water splitting by a dual-absorber tandem cell. Nature Photon. 6, 824–828 (2012).
Koo, B. et al. Hollow iron oxide nanoparticles for application in lithium ion batteries. Nano Lett. 12, 2429–2435 (2012).
Terris, B. D. & Thomson, T. Nanofabricated and self-assembled magnetic structures as data storage media. J. Phys. D 38, R199–R222 (2005).
Galvis, H. et al. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 335, 835–838 (2012).
Cundy, A. B., Hopkinson, L. & Whitby, R. L. D. Use of iron-based technologies in contaminated land and groundwater remediation: A review. Sci. Total Environ. 400, 42–51 (2008).
Gao, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotech. 2, 577–583 (2007).
Pankhurst, Q. A., Thanh, N. T. K., Jones, S. K. & Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D 42, 224001 (2009).
Valencia, P. M., Farokhzad, O. C., Karnik, R. & Langer, R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nature Nanotech. 7, 623–629 (2012).
Peng, S., Wang, C., Xie, J. & Sun, S. Synthesis and stabilization of monodisperse iron nanoparticles. J. Am. Chem. Soc. 128, 10676–10677 (2006).
Cheong, S. et al. Simple synthesis and functionalization of iron nanoparticles for magnetic resonance imaging. Angew. Chem. Int. Ed. 50, 4206–4209 (2011).
Binns, C. et al. Preparation of hydrosol suspensions of elemental and core-shell nanoparticles by co-deposition with water vapour from the gas-phase in ultra-high vacuum conditions. J. Nanopart. Res. 14, 1136 (2012).
LaGrow, A. P. et al. Synthesis, alignment and magnetic properties of monodisperse nickel nanocubes. J. Am. Chem. Soc. 134, 855–858 (2012).
Shavel, A., Rodrı´guez-González, B., Spasova, M., Farle, M. & Liz-Marzán, L. M. Synthesis and characterization of Fe/Fe oxide core/shell nanocubes. Adv. Funct. Mater. 17, 3870–3878 (2007).
Vystavel, T., Palasantzas, G., Koch, S. A. & De Hosson, J. Th. M. Nanosized iron clusters investigated with in situ transmission electron microscopy. Appl. Phys. Lett. 82, 197–199 (2003).
Pratt, A., Woffinden, C., Kröger, R., Tear, S. & Binns, C. Surface spin polarization of Fe nanoclusters. IEEE Trans. Mag. 46, 1660–1662 (2010).
Cabrera, N. & Mott, N. F. Theory of the oxidation of metals. Rep. Prog. Phys. 12, 163–184 (1949).
Yin, Y. et al. Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304, 711–714 (2004).
Cabot, A. et al. Vacancy coalescence during the oxidation of iron nanoparticles. J. Am. Chem. Soc. 129, 10358–10360 (2007).
Tang, J., Myers, M., Bosnick, K. A. & Brus, L. E. Magnetite Fe3O4 nanocrystals: Spectroscopic observation of aqueous oxidation kinetics. J. Phys. Chem. B 107, 7501–7506 (2003).
Wang, C. et al. Void formation during early stages of passivation: Initial oxidation of Fe nanoparticles at room temperature. J. Appl. Phys. 98, 094308 (2005).
Hÿtch, M. J., Putaux, J-L. & Pénisson, J-M. Measurement of the displacement field of dislocations to 0.03 Å by electron microscopy. Nature 423, 270–273 (2003).
Reichel, F., Juergens, L. P. H. & Mittemeijer, E. J. Thermodynamic model of oxide overgrowth on bare metals: Relaxation of growth strain by plastic deformation. Phys. Rev. B 74, 144103 (2006).
Mordehai, D., Kazakevich, M., Srolovitz, D. J. & Rabkin, E. Nanoindentation size effect in single-crystal nanoparticles and thin films: A comparative experimental and simulation study. Acta Matter. 59, 2309–2321 (2011).
Wang, C., Baer, D. R., Amonette, J. E., Engelhard, M. H., Anthony, J. & Qiang, Y. Morphology and electronic structure of the oxide shell on the surface of iron nanoparticles. J. Am. Chem. Soc. 131, 8824–8832 (2009).
Philibert, J. Atomic Movements—Diffusion and Mass Transport in Solids (Les Édition de Physique, 1991).
Gorski, W. S. Theorie der elastischen Nachwirkung in ungeordneten Mischkristallen zweiter. Phys. Z. Sowj. 8, 457–471 (1935).
Korte, C., Peters, A., Janek, J., Hesse, J. & Zakharov, N. Ionic conductivity and activation energy for oxygen ion transport in superlattices—the semicoherent multilayer system YSZ (ZrO2+9.5mol%Y2O3)/Y2O3 . Phys. Chem. Chem. Phys. 10, 4623–4635 (2008).
Acknowledgements
We acknowledge support by the Max-Kade foundation for a Visiting Professorship stipend to one of the authors (R.K.) as well as financial support by the World University Network (WUN) for the collaboration with the University of Illinois at Champaign-Urbana (R.K.). The Engineering and Physical Sciences Research Council (EPSRC) are also acknowledged for funding the initial stages of this project (EP/D034604/1). Electron microscopy was carried out at the Materials Research Laboratory Central Facilities at the University of Illinois at Urbana Champaign and the York JEOL Nanocentre. We are grateful to E. Rabkin for critically reading the manuscript. O.H. gratefully acknowledges support from a Marie Curie Intra European Fellowship within the 7th European Community Framework Programme under grant agreement PIEF-GA-2010-273014.
Author information
Authors and Affiliations
Contributions
R.K., A.P. and C.B. planned the initial experiments led by R.K. Deposition of Fe nanoparticles was carried out by A.P., C.W. and S.P.T. Electron microscopy and EELS were performed by A.S. and R.K. in Illinois and L.L. and R.K. in York. The atomic-level strain analysis procedure used was developed by R.K. with additional strain analysis by A.P. Theoretical models were developed analytically by O.H. and numerically by R.K. The manuscript was prepared by A.P. and R.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1264 kb)
Rights and permissions
About this article
Cite this article
Pratt, A., Lari, L., Hovorka, O. et al. Enhanced oxidation of nanoparticles through strain-mediated ionic transport. Nature Mater 13, 26–30 (2014). https://doi.org/10.1038/nmat3785
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3785
This article is cited by
-
ReaxFF molecular dynamics study of early oxidation of nickel nanoparticles
Journal of Materials Science (2024)
-
Synthesis and characterization of amorphous-nanocrystalline Fe70Cr10Nb10B10 powders by mechanical alloying
Applied Physics A (2022)
-
Collective dipole effects in ionic transport under electric fields
Nature Communications (2020)
-
Unexpected Kirkendall effect in twinned icosahedral nanocrystals driven by strain gradient
Nano Research (2020)
-
In situ TEM oxidation study of Fe thin-film transformation to single-crystal magnetite nanoparticles
Journal of Materials Science (2020)