Abstract
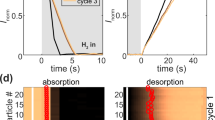
A quantitative understanding of nanocrystal phase transformations would enable more efficient energy conversion and catalysis, but has been hindered by difficulties in directly monitoring well-characterized nanoscale systems in reactive environments. We present a new in situ luminescence-based probe enabling direct quantification of nanocrystal phase transformations, applied here to the hydriding transformation of palladium nanocrystals. Our approach reveals the intrinsic kinetics and thermodynamics of nanocrystal phase transformations, eliminating complications of substrate strain, ligand effects and external signal transducers. Clear size-dependent trends emerge in nanocrystals long accepted to be bulk-like in behaviour. Statistical mechanical simulations show these trends to be a consequence of nanoconfinement of a thermally driven, first-order phase transition: near the phase boundary, critical nuclei of the new phase are comparable in size to the nanocrystal itself. Transformation rates are then unavoidably governed by nanocrystal dimensions. Our results provide a general framework for understanding how nanoconfinement fundamentally impacts broad classes of thermally driven solid-state phase transformations relevant to hydrogen storage, catalysis, batteries and fuel cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sear, R. P. Nucleation: Theory and applications to protein solutions and colloidal suspensions. J. Phys. Condens. Matter 19, 033101 (2007).
Berube, V., Radtke, G., Dresselhaus, M. & Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 31, 637–663 (2007).
Chen, C-C., Herhold, A. B., Johnson, C. S. & Alivisatos, A. P. Size dependence of structural metastability in semiconductor nanocrystals. Science 276, 398–401 (1997).
Lee, B-S. et al. Observation of the role of subcritical nuclei in crystallization of a glassy solid. Science 326, 980–984 (2009).
Oxtoby, D. W. Nucleation of first-order phase transitions. Acc. Chem. Res. 31, 91–97 (1998).
Langhammer, C., Zhdanov, V. P., Zorić, I. & Kasemo, B. Size-dependent kinetics of hydriding and dehydriding of Pd nanoparticles. Phys. Rev. Lett. 104, 135502 (2010).
Favier, F., Walter, E. C., Zach, M. P., Benter, T. & Penne, R. M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 293, 2227–2231 (2001).
Jeon, K-J. et al. Air-stable magnesium nanocomposites provide rapid and high-capacity hydrogen storage without using heavy-metal catalysts. Nature Mater. 10, 286–290 (2011).
Bardhan, R., Ruminski, A. M., Brand, A. & Urban, J. J. Magnesium nanocrystal-polymer composites: A new platform for designer hydrogen storage materials. Energy Environ. Sci. 4, 4882–4895 (2011).
Gremaud, R., Slaman, M., Schreuders, H., Dam, B. & Griessen, R. An optical method to determine the thermodynamics of hydrogen absorption and desorption in metals. Appl. Phys. Lett. 91, 231916 (2007).
Baldi, A., Gonzalez-Silveira, M., Palmisano, V., Dam, B. & Griessen, R. Destabilization of the Mg–H system through elastic constraints. Phys. Rev. Lett. 102, 226102 (2009).
Langhammer, C., Larsson, E. M., Kasemo, B. & Zorić, I. Indirect nanoplasmonic sensing: Ultrasensitive experimental platform for nanomaterials science and optical nanocalorimetry. Nano Lett. 10, 3529–3538 (2010).
Langhammer, C., Zorić, I., Kasemo, B. & Clemens, B. M. Hydrogen storage in Pd nanodisks characterized with a novel nanoplasmonic sensing scheme. Nano Lett. 7, 3122–3127 (2007).
Liu, N., Tang, M. L., Hentschel, M., Giessen, H. & Alivisatos, A. P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nature Mater. 10, 631–636 (2011).
Varnavski, O. P., Mohamed, M. B., El-Sayed, M. A. & Goodson, T. III Relative enhancement of ultrafast emission in gold nanorods. J. Phys. Chem. B 107, 3101–3104 (2003).
Fedorovich, R. D., Naumovets, A. G. & Tomchuk, P. M. Electron and light emission from island metal films and generation of hot electrons in nanoparticles. Phys. Rep. 328, 73–179 (2000).
Luther, J. M., Jain, P. K., Ewers, T. & Alivisatos, A. P. Localized surface plasmon resonances arising from free carriers in doped quantum dots. Nature Mater. 10, 361–366 (2011).
Eastman, D. E., Cashion, J. K. & Switendick, A. C. Photoemission studies of energy levels in the palladium–hydrogen system. Phys. Rev. Lett. 27, 35–38 (1971).
Yamauchi, M., Kobayashi, H. & Kitagawa, H. Hydrogen storage mediated by Pd and Pt nanoparticles. ChemPhysChem 10, 2566–2576 (2009).
Binney, J., Dowrick, N., Fisher, A. & Newman, M. The Theory of Critical Phenomena (Oxford Univ. Press, 1992).
Alefeld, G. & Völkl, J. Topics in Applied Physics: Hydrogen in Metals II: Application-Oriented Properties (Springer, 1978).
Buck, H. & Alefeld, G. Hydrogen in palladium–silver in the neighbourhood of the critical point. Phys. Stat. Sol. B 49, 317–327 (1972).
Langhammer, C., Zhdanov, V. P., Zorić, I. & Kasemo, B. Size-dependent hysteresis in the formation and decomposition of hydride in metal nanoparticles. Chem. Phys. Lett. 488, 62–66 (2010).
Salomons, E., Griessen, R., De Groot, D. G. & Magerl, A. Surface tension and subsurface sites of metallic nanocrystals determined from H-absorption. Europhys. Lett. 5, 449–454 (1988).
Wagner, H. & Horner, H. Elastic interaction and the phase transition in coherent metal-hydrogen systems. Adv. Phys. 23, 587–637 (1974).
Ten Wolde, P. R., Ruiz-Montero, M. J. & Frenkel, D. Numerical calculation of the rate of crystal nucleation in a Lennard-Jones system at moderate undercooling. J. Chem. Phys. 104, 9932–9947 (1996).
Zhdanov, V. P. & Kasemo, B. The formation of a new phase in nanoparticles. Physica E 41, 775–778 (2009).
Zhdanov, V. P. & Kasemo, B. Kinetics of the formation of a new phase in nanoparticles. Chem. Phys. Lett. 460, 158–161 (2008).
Pundt, A. & Kirchheim, R. Hydrogen in metals: Microstructural aspects. Annu. Rev. Mater. Res. 36, 555–608 (2006).
Binder, K. & Landau, D. P. Finite-size scaling at first-order phase transitions. Phys. Rev. B 30, 1477–1485 (1984).
Furukawa, H. & Binder, K. Two-phase equilibria and nucleation barriers near a critical point. Phys. Rev. A 26, 556–566 (1982).
Rikvold, P. A., Tomita, H., Miyashita, S. & Sides, S. W. Metastable lifetimes in a kinetic Ising model: Dependence on field and system size. Phys. Rev. E 49, 5080–5090 (1994).
Dura, J. A. et al. Porous Mg formation upon dehydrogenation of MgH2 thin films. J. Appl. Phys. 109, 093501 (2011).
Olsson, S. & Hjörvarsson, B. Effect of biaxial elastic constraints on H-H interactions in ultrathin vanadium. Phys. Rev. B 71, 035414 (2005).
Olsson, S., Blixt, A. M. & Hjörvarsson, B. Mean-field-like structural phase transition of H in Fe/V(001) superlattices. J. Phys. Condens. Matter 17, 2073–2084 (2005).
Pálsson, G. K. et al. Hydrogen site occupancy and strength of forces in nano-sized metal hydrides. Phys. Rev. B 85, 195407 (2012).
De Ribaupierre, Y. & Manchester, F. D. Experimental study of the critical-point behaviour of the hydrogen in palladium system. I. Lattice gas aspects. J. Phys. C 7, 2126–2139 (1974).
Jongh, P. E. d. & Adelhel, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. Chem. Sus. Chem. 3, 1332–1348 (2010).
Niu, W., Zhang, L. & Xu, G. Shape-controlled synthesis of single-crystalline palladium nanocrystals. ACS Nano 4, 1987–1996 (2010).
Hedges, L. O. & Whitelam, S. Patterning a surface so as to speed nucleation from solution. Soft Matter 8, 8624–8635 (2012).
Acknowledgements
We would like to thank R. Hauge at Rice University, Houston, Texas for help with the optical cell. We would also like to acknowledge A. Schwartzberg, T. Mattox, R. Buonsanti, A. Ruminski, T. Kyukendall, I. Tamblyn and L. Maibaum for helpful discussions. Work at the Molecular Foundry was supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. J.J.U. and R.B. are supported under the US Department of Energy Hydrogen Storage Program, B&R code KC0202020. C.L.P. and A.J. are supported under Mohr Davidow Ventures and Berkeley Sensor and Actuators Center. L.O.H. was supported by the Center for Nanoscale Control of Geologic CO2, a US D.O.E. Energy Frontier Research Center, under Contract No. DE-AC02–05CH11231.
Author information
Authors and Affiliations
Contributions
J.J.U. and R.B. conceived and designed the experiments. R.B. synthesized and characterized the nanoparticles and performed the experiments. C.L.P. designed and built the optical cell; C.L.P. and R.B. built the high-vacuum gas-flow system. L.O.H. and S.W. conceived the simulation protocol, and L.O.H. carried out the simulations. R.B., L.O.H., S.W. and J.J.U. analysed the data and wrote the manuscript. A.J., S.W. and J.J.U. discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4157 kb)
Rights and permissions
About this article
Cite this article
Bardhan, R., Hedges, L., Pint, C. et al. Uncovering the intrinsic size dependence of hydriding phase transformations in nanocrystals. Nature Mater 12, 905–912 (2013). https://doi.org/10.1038/nmat3716
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3716
This article is cited by
-
Fine-tuning of Pd-Rh core-shell catalysts by interstitial hydrogen doping for enhanced methanol oxidation
Nano Research (2022)
-
Structure of a seeded palladium nanoparticle and its dynamics during the hydride phase transformation
Communications Chemistry (2021)
-
Simulations of oxidation of metal nanoparticles with a grain boundary inside
Reaction Kinetics, Mechanisms and Catalysis (2020)
-
Facets and vertices regulate hydrogen uptake and release in palladium nanocrystals
Nature Materials (2019)
-
Thermally-induced reversible structural isomerization in colloidal semiconductor CdS magic-size clusters
Nature Communications (2018)