Abstract
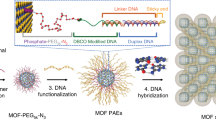
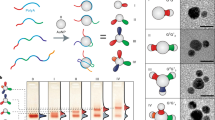
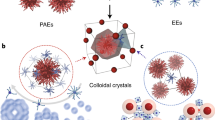
Nanoparticles can be combined with nucleic acids to programme the formation of three-dimensional colloidal crystals where the particles’ size, shape, composition and position can be independently controlled1,2,3,4,5,6,7. However, the diversity of the types of material that can be used is limited by the lack of a general method for preparing the basic DNA-functionalized building blocks needed to bond nanoparticles of different chemical compositions into lattices in a controllable manner. Here we show that by coating nanoparticles protected with aliphatic ligands with an azide-bearing amphiphilic polymer, followed by the coupling of DNA to the polymer using strain-promoted azide–alkyne cycloaddition8 (also known as copper-free azide–alkyne click chemistry), nanoparticles bearing a high-density shell of nucleic acids can be created regardless of nanoparticle composition. This method provides a route to a virtually endless class of programmable atom equivalents for DNA-based colloidal crystallization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mirkin, C. A., Letsinger, R. L., Mucic, R. C. & Storhoff, J. J. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996).
Mirkin, C. A. The polyvalent gold nanoparticle conjugate-materials synthesis, biodiagnostics, and intracellular gene regulation. Mater. Res. Soc. Bull. 35, 532–539 (2010).
Park, S. Y. et al. DNA-programmable nanoparticle crystallization. Nature 451, 553–556 (2008).
Nykypanchuk, D., Maye, M. M., van der Lelie, D & Gang, O. DNA-guided crystallization of colloidal nanoparticles. Nature 451, 549–552 (2008).
Macfarlane, R. J. et al. Nanoparticle superlattice engineering with DNA. Science 334, 204–208 (2011).
Maye, M. M., Kumara, M. T., Nykypanchuk, D., Sherman, W. B. & Gang, O. Switching binary states of nanoparticle superlattices and dimer clusters by DNA strands. Nature Nanotech. 5, 116–120 (2010).
Jones, M. R. et al. DNA-nanoparticle superlattices formed from anisotropic building blocks. Nature Mater. 9, 913–917 (2010).
Agard, N. J., Prescher, J. A. & Bertozzi, C. R. A strain-promoted 3+2 azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937 (1996).
Cutler, J. I., Auyeung, E. & Mirkin, C. A. Spherical nucleic acids. J. Am. Chem. Soc. 134, 1376–1391 (2012).
Daniel, M. C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Jeong, U., Teng, X., Wang, Y., Yang, H. & Xia, Y. Superparamagnetic colloids: Controlled synthesis and niche applications. Adv. Mater. 19, 33–60 (2007).
Sun, S. H. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv. Mater. 18, 393–403 (2006).
Murray, C. B., Kagan, C. R. & Bawendi, M. G. Synthesis and characterization of monodisperse nanocrystals and close-packed nanocrystal assemblies. Annu. Rev. Mater. Sci. 30, 545–610 (2000).
Talapin, D. V., Lee, J. S., Kovalenko, M. V. & Shevchenko, E. V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 110, 389–458 (2010).
Macfarlane, R. J. et al. Establishing the design rules for DNA-mediated colloidal crystallization. Angew. Chem. Int. Ed. 49, 4589–4592 (2010).
Cutler, J. I., Zheng, D., Xu, X. Y., Giljohann, D. A. & Mirkin, C. A. Polyvalent oligonucleotide iron oxide nanoparticle ‘Click’ conjugates. Nano Lett. 10, 1477–1480 (2010).
Sun, D. Z. & Gang, O. Binary heterogeneous superlattices assembled from quantum dots and gold nanoparticles with DNA. J. Am. Chem. Soc. 133, 5252–5254 (2011).
Tikhomirov, G. et al. DNA-based programming of quantum dot valency, self-assembly and luminescence. Nature Nanotech. 6, 485–490 (2011).
Gao, X. H., Cui, Y. Y., Levenson, R. M., Chung, L. W. K. & Nie, S. M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 22, 969–976 (2004).
Pellegrino, T. et al. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: A general route to water soluble nanocrystals. Nano Lett. 4, 703–707 (2004).
Di Corato, R. et al. Water solubilization of hydrophobic nanocrystals by means of poly(maleic anhydride-alt-1-octadecene). J. Mater. Chem. 18, 1991–1996 (2008).
Shtykova, E. V. et al. Hydrophilic monodisperse magnetic nanoparticles protected by an amphiphilic alternating copolymer. J. Phys. Chem. C 112, 16809–16817 (2008).
Lin, C. A. J. et al. Design of an amphiphilic polymer for nanoparticle coating and functionalization. Small 4, 334–341 (2008).
Yu, W. W. et al. Forming biocompatible and non-aggregated nanocrystals in water using amphiphilic polymers. J. Am. Chem. Soc. 129, 2871–2879 (2007).
Jin, R., Wu, G., Li, Z., Mirkin, C. A. & Schatz, G. C. What controls the melting properties of DNA-linked gold nanoparticle assemblies? J. Am. Chem. Soc. 125, 1643–1654 (2003).
Macfarlane, R. J. et al. Assembly and organization processes in DNA-directed colloidal crystallization. Proc. Natl Acad. Sci. USA 106, 10493–10498 (2009).
Elghanian, R., Storhoff, J. J., Mucic, R. C., Letsinger, R. L. & Mirkin, C. A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 277, 1078–1081 (1997).
Hurst, S. J., Lytton-Jean, A. K. R. & Mirkin, C. A. Maximizing DNA loading on a range of gold nanoparticle sizes. Anal. Chem. 78, 8313–8318 (2006).
Hill, H. D. et al. Controlling the lattice parameters of gold nanoparticle FCC crystals with duplex DNA linkers. Nano Lett. 8, 2341–2344 (2008).
Auyeung, E., Macfarlane, R. J., Choi, C. H. J., Cutler, J. I. & Mirkin, C. A. Transitioning DNA-engineered nanoparticle superlattices from solution to the solid state. Adv. Mater. 24, 5181–5186 (2012).
Acknowledgements
C.A.M. acknowledges the support of DoD/NSSEFF/NPS Awards N00244-09-1-0012 and N00244-09-1-0071, AFOSR Awards FA9550-11-1-0275, FA9550-12-1-0280, and FA9550-09-1-0294, the National Science Foundation’s MRSEC programme (DMR-0520513) at the Materials Research Center of Northwestern University, the Defense Advanced Research Projects Agency (DARPA)/Microsystems Technology Office (MTO) under Award Nos HR0011-13-2-0002, HR0011-13-2-0018 and N66001-11-1-4189, and the Non-equilibrium Energy Research Center (NERC), an Energy Frontier Research Center funded by the US DOE, Office of Science, Office of Basic Energy Sciences Award DE-SC0000989 (nanoparticle synthesis). Any opinions, findings, and conclusion or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the funding agencies, and no official endorsement should be inferred. R.J.M. acknowledges a Ryan Fellowship from Northwestern University. K.L.Y. and E.A. acknowledge National Defense Science and Engineering Graduate Research Fellowships. C.H.J.C. acknowledges a postdoctoral research fellowship from the Croucher Foundation. L.H. acknowledges the HHMI for support from an international student research fellowship. SAXS experiments were carried out at the Dupont–Northwestern–Dow Collaborative Access Team beam line at the Advanced Photon Source (APS), Argonne National Laboratory, and use of the APS was supported by the DOE (DE-AC02-06CH11357). The electron microscopy work was performed at the Biological Imaging Facility (BIF) and the Electron Probe Instrumentation Center (EPIC) at Northwestern University.
Author information
Authors and Affiliations
Contributions
C.Z., R.J.M. and C.A.M. initiated the concepts. C.Z. and R.J.M. designed the experiments. C.Z. conducted the experiments. C.Z., R.J.M. and C.A.M. wrote the manuscript. All authors contributed to data collection, data analysis and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4092 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Macfarlane, R., Young, K. et al. A general approach to DNA-programmable atom equivalents. Nature Mater 12, 741–746 (2013). https://doi.org/10.1038/nmat3647
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3647
This article is cited by
-
Supercrystal engineering of atomically precise gold nanoparticles promoted by surface dynamics
Nature Chemistry (2023)
-
Spherical Packing Superlattices in Self-Assembly of Homogenous Soft Matter: Progresses and Potentials
Chinese Journal of Polymer Science (2023)
-
Phase transferring luminescent gold nanoclusters via single-stranded DNA
Science China Chemistry (2022)
-
PolyA-based DNA bonds with programmable bond length and bond energy
NPG Asia Materials (2020)
-
Colloidal crystal engineering with metal–organic framework nanoparticles and DNA
Nature Communications (2020)