Abstract
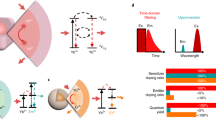
Three-photon excitation is a process that occurs when three photons are simultaneously absorbed within a luminophore for photo-excitation through virtual states. Although the imaging application of this process was proposed decades ago, three-photon biomedical imaging has not been realized yet owing to its intrinsic low quantum efficiency. We herein report on high-resolution in vitro and in vivo imaging by combining three-photon excitation of ZnS nanocrystals and visible emission from Mn2+ dopants. The large three-photon cross-section of the nanocrystals enabled targeted cellular imaging under high spatial resolution, approaching the theoretical limit of three-photon excitation. Owing to the enhanced Stokes shift achieved through nanocrystal doping, the three-photon process was successfully applied to high-resolution in vivo tumour-targeted imaging. Furthermore, the biocompatibility of ZnS nanocrystals offers great potential for clinical applications of three-photon imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Zipfel, W. R., Williams, R. M. & Webb, W. W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nature Biotechnol. 21, 1369–1377 (2003).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. K. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 22, 969–976 (2004).
Kim, S. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 22, 93–97 (2004).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 307, 538–544 (2005).
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Mater. 4, 435–446 (2005).
Scholes, G. D. & Rumbles, G. Excitons in nanoscale systems. Nature Mater. 5, 683–696 (2006).
Larson, D. R. et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo. Science 300, 1434–1436 (2003).
Stroh, M. et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nature Med. 11, 678–682 (2005).
Voura, E. B., Jaiswal, J. K., Mattoussi, H. & Simon, S. M. Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nature Med. 10, 993–998 (2004).
Choi, H. S. & Frangioni, J. V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging 9, 291–310 (2010).
Bhargava, R. N., Gallagher, D., Hong, X. & Nurmikko, A. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 72, 416–419 (1994).
Sooklal, K., Cullum, B. S., Angel, S. M. & Murphy, C. J. Photophysical properties of ZnS nanoclusters with spatially localized Mn2+. J. Phys. Chem. 100, 4551–4555 (1996).
Suyver, J. F., Wuister, S. F., Kelly, J. J. & Meijerink, A. Synthesis and photoluminescence of nanocrystalline ZnS: Mn2+. Nano Lett. 1, 429–433 (2001).
Srivastava, B. B. et al. Highly luminescent Mn-doped ZnS nanocrystals: Gram-scale synthesis. J. Phys. Chem. Lett. 1, 1454–1458 (2010).
Deng, Z. et al. High-quality manganese-doped zinc sulfide quantum rods with tunable dual-color and multiphoton emissions. J. Am. Chem. Soc. 133, 5389–5396 (2011).
Manzoor, K. et al. Bio-conjugated luminescent quantum dots of doped ZnS: A cyto-friendly system for targeted cancer imaging. Nanotechnology 20, 065102 (2009).
Norris, D. J., Efros, A. L. & Erwin, S. C. Doped nanocrystals. Science 319, 1776–1779 (2008).
Bryan, J. D. & Gamelin, D. R. Doped semiconductor nanocrystals: Synthesis, characterization, physical properties and applications. Prog. Inorg. Chem. 54, 47–126 (2005).
Erwin, S. C. et al. Doping semiconductor nanocrystals. Nature 436, 91–94 (2005).
Yang, Y., Chen, O., Angerhofer, A. & Cao, Y. C. On doping CdS/ZnS core/shell nanocrystals with Mn. J. Am. Chem. Soc. 130, 15649–15661 (2008).
Bussian, D. A. et al. Tunable magnetic exchange interactions in manganese-doped inverted core–shell ZnSe–CdSe nanocrystals. Nature Mater. 8, 35–40 (2009).
Beaulac, R., Schneider, L., Archer, P. I., Bacher, G. & Gamelin, D. R. Light-induced spontaneous magnetization in doped colloidal quantum dots. Science 325, 973–976 (2009).
Yu, J. H. et al. Giant Zeeman splitting in nucleation-controlled doped CdSe: Mn2+ quantum nanoribbons. Nature Mater. 9, 47–53 (2010).
Hell, S. W. et al. Three-photon excitation in fluorescence microscopy. J. Biomed. Opt. 1, 71–74 (1996).
Wokosin, D. L., Centonze, V. E., Crittenden, S. & White, J. Three-photon excitation fluorescence imaging of biological specimens using an all-solid-state laser. Bioimaging 4, 208–214 (1996).
Xu, C., Zipfel, W., Shear, J. B., Williams, R. M. & Webb, W. W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl Acad. Sci. USA 93, 10763–10768 (1996).
Maiti, S., Shear, J. B., Williams, R. M., Zipfel, W. R. & Webb, W. W. Measuring serotonin distribution in live cells with three-photon excitation. Science 275, 530–532 (1997).
He, G. S., Markowicz, P. P., Lin, T-C. & Prasad, P. N. Observation of stimulated emission by direct three-photon excitation. Nature 415, 767–770 (2002).
Chon, J. W. M., Gu, M., Bullen, C. & Mulvaney, P. Three-photon excited band edge and trap emission of CdS semiconductor nanocrystals. Appl. Phys. Lett. 84, 4472–4474 (2004).
He, J., Ji, W., Mi, J., Zheng, Y. G. & Ying, J. Y. Three-photon absorption in water-soluble ZnS nanocrystals. Appl. Phys. Lett. 88, 181114 (2006).
He, J. et al. Direct observation of three-photon resonance in water-soluble ZnS quantum dots. Appl. Phys. Lett. 92, 131114 (2008).
Xing, G., Ji, W., Zheng, Y. & Ying, J. Y. High efficiency and nearly cubic power dependence of below-band-edge photoluminescence in water-soluble, copper-doped ZnSe/ZnS quantum dots. Opt. Express 16, 5710–5715 (2008).
Zipfel, W. R. et al. Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl Acad. Sci. USA 100, 7075–7080 (2003).
Dubertret, B. et al. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762 (2002).
Chen, H-Y., Chen, T-Y. & Son, D. H. Measurement of energy transfer time in colloidal Mn-doped semiconductor nanocrystals. J. Phys. Chem. C 114, 4418–4423 (2010).
Ruouslahti, E. Specialization of tumour vasculature. Nature Rev. Cancer 2, 83–90 (2002).
Fogal, V., Zhang, L., Krajewski, S. & Rouslahti, E. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res. 68, 7210–7218 (2008).
Derfus, A. M., Chan, W. C. W. & Bhatia, S. N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 4, 11–18 (2004).
Lewinski, N., Colvin, V. & Drezek, R. Cytotoxicity of nanoparticles. Small 4, 26–49 (2008).
Centonze, V. E. & White, J. G. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys. J. 75, 2015–2024 (1998).
Cai, W. et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 6, 669–676 (2006).
Smith, B. R. et al. Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett. 8, 2599–2606 (2008).
Lansford, R., Bearman, G. & Fraser, S. E. Resolution of multiple green fluorescent protein color variants and dyes using two-photon microscopy and imaging spectroscopy. J. Biomed. Opt. 6, 311–318 (2001).
Park, J. H. et al. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nature Mater. 8, 331–336 (2009).
Hauck, T. S., Anderson, R. E., Fischer, H. C., Newbigging, S. & Chan, W. C. W. In vivo quantum-dot toxicity assessment. Small 6, 138–144 (2010).
Acknowledgements
We thank A. Lutich (Ludwig-Maximilians-Universtität München) for a preliminary study on multi-photon spectroscopy and W. Ji (The National University of Singapore) for helpful discussions on the three-photon excitation mechanism. We thank M-S. Won at the Korea Basic Science Institute (KBSI) for the electron paramagnetic resonance characterization, and S. Her at KBSI for the review and suggestion of animal experiment design. We acknowledge financial support by the Research Center Program of Institute for Basic Science (IBS) in Korea.
Author information
Authors and Affiliations
Contributions
J.H.Y., S-H.K. and T.H. designed and carried out the experiments, analysed the data and wrote the manuscript. Z.P. and P.S. carried out the FCS study and interpreted the data. S-H.K., O.K.P., J.H.Y. and J.H.K. designed and carried out the multiphoton imaging experiments. J.H.Y., S.W.J., K.S. and D.W.L. carried out the synthesis of the materials. J.H.Y., K.S., M.C. and Y.I.P. carried out the bioconjugation of the NCs. S-H.K., O.K.P. and K.P. carried out the animal experiments. S-H.K., O.K.P., H.B.N. and N.L. carried out the in vitro toxicity evaluation. All authors have reviewed, discussed and approved the results and conclusions of this Article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2264 kb)
Supplementary Information
Supplementary Movie S1 (AVI 904 kb)
Rights and permissions
About this article
Cite this article
Yu, J., Kwon, SH., Petrášek, Z. et al. High-resolution three-photon biomedical imaging using doped ZnS nanocrystals. Nature Mater 12, 359–366 (2013). https://doi.org/10.1038/nmat3565
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3565
This article is cited by
-
Durable organic nonlinear optical membranes for thermotolerant lightings and in vivo bioimaging
Nature Communications (2023)
-
Multiphoton excited singlet/triplet mixed self-trapped exciton emission
Nature Communications (2023)
-
Mn2+-activated dual-wavelength emitting materials toward wearable optical fibre temperature sensor
Nature Communications (2022)
-
DFT calculations of 2D graphene like ZnS:Mn sheet for RESOLFT microscopic applications
Journal of Computational Electronics (2022)
-
In situ NMR reveals real-time nanocrystal growth evolution via monomer-attachment or particle-coalescence
Nature Communications (2021)