Abstract
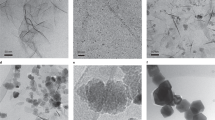
The formation of crystalline materials from solution is usually described by the nucleation and growth theory, where atoms or molecules are assumed to assemble directly from solution1. For numerous systems, the formation of the thermodynamically stable crystalline phase is additionally preceded by metastable intermediates 2. More complex pathways have recently been proposed, such as aggregational processes of nanoparticle precursors or pre-nucleation clusters, which seem to contradict the classical theory3,4,5,6. Here we show by cryogenic transmission electron microscopy that the nucleation and growth of magnetite—a magnetic iron oxide with numerous bio- and nanotechnological applications7—proceed through rapid agglomeration of nanometric primary particles and that in contrast to the nucleation of other minerals5, no intermediate amorphous bulk precursor phase is involved. We also demonstrate that these observations can be described within the framework of classical nucleation theory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kashchiev, D. Nucleation: Theory and Basic Applications (Butterworth-Heinemann, 2000).
Ostwald, W. Studien uber die Bildung und Umwandlung fester Korper. Z. Phys. Chem. 22, 289–330 (1897).
Banfield, J. F., Welch, S. A., Zhang, H., Thomsen Ebert, T. & Lee Penn, R. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 289, 751–754 (2000).
Navrotsky, A. Energetic clues to pathways to biomineralization: Precursors, clusters, and nanoparticles. Proc. Natl Acad. Sci. USA 101, 12096–12101 (2004).
Gebauer, D., Volkel, A. & Cölfen, H. Stable prenucleation calcium carbonate clusters. Science 322, 1819–1822 (2008).
Pouget, E. M. et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 323, 1455–1458 (2009).
Laurent, S. et al. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 108, 2064–2110 (2008).
Coey, J. M. D. & Chien, C. L. Half-metallic ferromagnetic oxides. Mater. Res. Bull. 28, 720–724 (2003).
Cölfen, H. & Antonietti, M. Mesocrystals: Inorganic superstructures made by highly parallel crystallization and controlled alignment. Angew. Chem. Int. Ed. 44, 5576–5591 (2005).
Hu, Q. et al. The thermodynamics of calcite nucleation at organic interfaces: Classical vs. non-classical pathways. Faraday Discuss. 159, 509–523 (2012).
Yuwono, V. M., Burrows, N. D., Soltis, J. A. & Penn, R. L. Oriented aggregation: Formation and transformation of mesocrystal intermediates revealed. J. Am. Chem. Soc. 132, 2163–2165 (2010).
Van Driessche, A. E. S. et al. The role and implications of bassanite as a stable precursor phase to gypsum precipitation. Science 336, 69–72 (2012).
Ge, J., Hu, Y., Biasini, M., Beyermann, W. P. & Yin, Y. Superparamagnetic magnetite colloidal nanocrystal clusters. Angew. Chem. Int. Ed. 46, 4342–4345 (2007).
Cornell, R. M. & Schwertmann, U. The Iron Oxides (Structure, Properties, Reactions, Occurrences and Uses) (Wiley-VCH, 2003).
Faivre, D. & Schüler, D. Magnetotactic bacteria and magnetosomes. Chem. Rev. 108, 4875–4898 (2008).
Lowenstam, H. A. Lepidocrocite an apatite mineral and magnetite in teeth of chitons (Polyplacophora). Science 156, 1373–1375 (1967).
Diebel, C. E., Proksch, R., Green, C. R., Neilson, P. & Walker, M. M. Magnetite defines a vertebrate magnetoreceptor. Nature 406, 299–302 (2000).
Mann, S., Sparks, N. H. C., Couling, S. B. & Larcombe, M. C. Crystallochemical characterization of magnetic spinels prepared from aqueous solution. J. Chem. Soc. 85, 3033–3044 (1989).
Blesa, M. A. & Matijevic, E. Phase-transformations of iron-oxides, oxohydroxides, and hydrous oxides in aqueous-media. Adv. Colloid Interface Sci. 29, 173–221 (1989).
Jolivet, J. P., Belleville, P., Tronc, E. & Livage, J. Influence of Fe(II) on the formation of the spinel iron-oxide in alkaline-medium. Clay Clay Min. 40, 531–539 (1992).
Faivre, D. et al. Mineralogical and isotopic properties of inorganic nanocrystalline magnetites. Geochim. Cosmochim. Acta 68, 4395–4403 (2004).
Dey, A. et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nature Mater. 9, 1010–1014 (2010).
Flynn, C. M. J. Hydrolysis of inorganic Iron(III) salts. Chem. Rev. 84, 31–41 (1984).
Hellman, H. et al. Identification of hydrolysis products of FeCl3.6H2O by ESI-MS. J. Mass Spectrom. 41, 1421–1429 (2006).
Tronc, E., Belleville, P., Jolivet, J. P. & Livage, J. Transformation of ferric hydroxide into spinel by Fe(II) adsorption. Langmuir 8, 313–319 (1992).
Fratzl, P., Lebowitz, J. L., Penrose, O. & Amar, J. Scaling functions, self-similarity, and the morphology of phase-separating systems. Phys. Rev. B 44, 4794–4811 (1991).
Mullin, J. W. Crystallization 3rd edn (Butterworth-Heinemann, 1992).
Ziemniak, S. E., Jones, M. E. & Combs, K. E. S. Magnetite solubility and phase stability in alkaline media at elevated temperatures. J. Solut. Chem. 24, 837–877 (1995).
Navrotsky, A., Ma, C., Lilova, K. & Birkner, N. Nanophase transition metal oxides show large thermodynamically driven shifts in oxidation-reduction equilibria. Science 330, 199–201 (2010).
Navrotsky, A. Nanoscale effects on thermodynamics and phase equilibria in oxide systems. ChemPhysChem 12, 2207–2215 (2011).
Pinney, N., Kubicki, J. D., Middlemiss, D. S., Grey, C. P. & Morgan, D. Density functional theory study of ferrihydrite and related Fe-oxyhydroxides. Chem. Mater. 21, 5727–5742 (2009).
Acknowledgements
H. Runge, R. Pitschke, S. Siegel and C. Li are acknowledged for technical assistance with electron microscopy and at the synchrotron. F. Nudelman helped prepare cryo-TEM samples. We thank E. Zolotoyabko, J. De Yoreo and M. Antonietti for discussions. This research was supported in D.F’s laboratory by the Max Planck Society, and a starting grant from the ERC (Project MB2, no. 256915).
Author information
Authors and Affiliations
Contributions
J.B. and C.L.C. carried out precipitation experiments. J.B. performed X-ray diffraction, TEM, analysed data and wrote the manuscript. P.H.H.B. performed cryo-TEM. A.D. carried out ferrihydrite experiments, performed cryo-TEM and analysed data. P.F. and J.B. developed the model. P.F., N.A.J.M.S. and D.F. supervised the project, analysed data and wrote the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
Max Planck Innovation has applied for the following patent: J. Baumgartner & D. Faivre, Process for preparing magnetite or maghemite nanoparticles with controlled size using mild conditions, international patent’s application number WO2010EP03983 priority date July 1st, 2010.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1390 kb)
Rights and permissions
About this article
Cite this article
Baumgartner, J., Dey, A., Bomans, P. et al. Nucleation and growth of magnetite from solution. Nature Mater 12, 310–314 (2013). https://doi.org/10.1038/nmat3558
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3558
This article is cited by
-
Impact of molecular symmetry on crystallization pathways in highly supersaturated KH2PO4 solutions
Nature Communications (2024)
-
Crystal dissolution by particle detachment
Nature Communications (2023)
-
Response surface method optimization of synthesized superparamagnetic magnetite (Fe3O4) nanoparticles characterized by pulsed IR calibration-free laser-induced breakdown spectroscopy
Applied Physics A (2023)
-
Fe3O4 nanoparticles synthesized by one-step reduction with nanoscale size-dependent magnetic properties
Journal of Sol-Gel Science and Technology (2023)
-
Surface-ligand-induced crystallographic disorder–order transition in oriented attachment for the tuneable assembly of mesocrystals
Nature Communications (2022)