Abstract
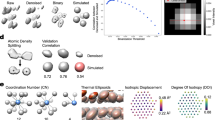
Distinct from inert bulk gold, nanoparticulate gold has been found to possess remarkable catalytic activity towards oxidation reactions. The catalytic performance of nanoparticulate gold strongly depends on size and support, and catalytic activity usually cannot be observed at characteristic sizes larger than 5 nm. Interestingly, significant catalytic activity can be retained in dealloyed nanoporous gold (NPG) even when its feature lengths are larger than 30 nm. Here we report atomic insights of the NPG catalysis, characterized by spherical-aberration-corrected transmission electron microscopy (TEM) and environmental TEM. A high density of atomic steps and kinks is observed on the curved surfaces of NPG, comparable to 3–5 nm nanoparticles, which are stabilized by hyperboloid-like gold ligaments. In situ TEM observations provide compelling evidence that the surface defects are active sites for the catalytic oxidation of CO and residual Ag stabilizes the atomic steps by suppressing {111} faceting kinetics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




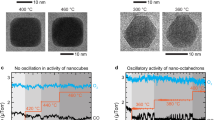
 under catalytic reactions for CO oxidation.
under catalytic reactions for CO oxidation.
 in pure gas environments using environmental TEM.
in pure gas environments using environmental TEM.
Similar content being viewed by others
References
Hvolbæk, B. et al. Catalytic activity of Au nanoparticles. Nano Today 2, 14–18 (2007).
Haruta, M., Kobayashi, T., Sano, H. & Yamada, N. Novel gold catalysts for the oxidation of carbon-monoxide at a temperature far below 0 °C. Chem. Lett. 16, 405–408 (1987).
Valden, M., Lai, X. & Goodman, D. W. Onset of catalytic activity of gold clusters on titania with the appearance of nonmetallic properties. Science 281, 1647–1650 (1998).
Hughes, M. D. et al. Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 437, 1132–1135 (2005).
Baker, T. A., Liu, X. Y. & Friend, C. M. The mystery of gold’s chemical activity: local bonding, morphology and reactivity of atomic oxygen. Phys. Chem. Chem. Phys. 13, 34–46 (2011).
Green, I. X., Tang, W. J., Neurock, M. & Yates, J. T. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 333, 736–739 (2011).
Wittstock, A., Zielasek, V., Biener, J., Friend, C. M. & Bäumer, M. Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327, 319–322 (2010).
Xu, C. X. et al. Low temperature CO oxidation over unsupported nanoporous gold. J. Am. Chem. Soc. 129, 42–43 (2007).
Asao, N. et al. Nanostructured materials as catalysts: Nanoporous-gold-catalyzed oxidation of organosilanes with water. Angew. Chem. Int. Edn. 49, 10093–10095 (2010).
Zielasek, V. et al. Gold catalysts: Nanoporous gold foams. Angew. Chem. Int. Edn. 45, 8241–8244 (2006).
Erlebacher, J., Aziz, M. J., Karma, A., Dimitrov, N. & Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001).
Biener, J. et al. Surface-chemistry-driven actuation in nanoporous gold. Nature Mater. 8, 47–51 (2009).
Snyder, J., Fujita, T., Chen, M. W. & Erlebacher, J. Oxygen reduction in nanoporous metal-ionic liquid composite electrocatalysts. Nature Mater. 9, 904–907 (2010).
Lang, X. Y., Hirata, A., Fujita, T. & Chen, M. W. Nanoporous metal/oxide hybrid electrodes for electrochemical supercapacitors. Nature Nanotech. 6, 232–236 (2011).
Ding, Y. & Chen, M.W. Nanoporous metals for catalytic and optical applications. MRS Bull. 34, 569–576 (2009).
Johnson, C. L. et al. Effects of elastic anisotropy on strain distributions in decahedral gold nanoparticles. Nature Mater. 7, 120–124 (2008).
Fujita, T., Qian, L. H., Inoke, K., Erlebacher, J. & Chen, M. W. Three-dimensional morphology of nanoporous gold. Appl. Phys. Lett. 92, 251902 (2008).
Tian, N., Zhou, Z. Y., Sun, S. G., Ding, Y. & Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316, 732–735 (2007).
Foiles, S. M., Baskes, M. I. & Daw, M. S. Embedded-atom-method functions for the fcc metals Cu, Ag, Au, Ni, Pd, Pt, and their alloys. Phys. Rev. B 33, 7983–7991 (1986).
Moskaleva, L. V. et al. Silver residues as a possible key to a remarkable oxidative catalytic activity of nanoporous gold. Phys. Chem. Chem. Phys. 13, 4529–4539 (2011).
Jiang, Q., Lu, H. M. & Zhao, M. Modelling of surface energies of elemental crystals. J. Phys. Condens. Matter 16, 521–530 (2004).
Hÿtch, M. J., Putaux, J. L. & Pénisson, J. M. Measurement of the displacement field of dislocations to 0.03 angstrom by electron microscopy. Nature 423, 270–273 (2003).
Galindo, P. L. et al. The Peak Pairs algorithm for strain mapping from HRTEM images. Ultramicroscopy 107, 1186–1193 (2007).
Crowson, D. A., Farkas, D. & Corcoran, S. G. Mechanical stability of nanoporous metals with small ligament sizes. Scr. Mater. 61, 497–499 (2009).
Lemire, C., Meyer, R., Shaikhutdinov, S. & Freund, H-J. Do quantum size effects control CO adsorption on gold nanoparticles? Angew. Chem. Int. Edn. 43, 118–121 (2003).
Lopez, N. et al. On the origin of the catalytic activity of gold nanoparticles for low-temperature CO oxidation. J. Catal. 223, 232–235 (2004).
Molina, L. M. & Hammer, B. Theoretical study of CO oxidation on Au nanoparticles supported by MgO(100). Phys. Rev. B 69, 155424 (2004).
Boudart, M. Turnover rates in heterogeneous catalysis. Chem. Rev. 95, 661–666 (1995).
McKenna, K. P. Gold nanoparticles under gas pressure. Phys. Chem. Chem. Phys. 11, 4145–4151 (2009).
Wittstock, A., Biener, J. & Baumer, M. Nanoporous gold: A new material for catalytic and sensor applications. Phy. Chem. Chem. Phys. 12, 12919–12930 (2010).
Frenken, J. W. M. & Stoltze, P. Are vicinal metal surfaces Stable? Phys. Rev. Lett. 82, 3500–3503 (1999).
Combe, N., Jensen, P. & Pimpinelli, A. Changing shapes in the nanoworld. Phys. Rev. Lett. 85, 110–113 (2000).
Guan, L. et al. Relaxation and electronic states of Au(100), (110) and (111) surfaces. Solid State Commun. 149, 1561–1564 (2009).
Mavrikakis, M., Hammer, B. & Norskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 81, 2819–2822 (1998).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core–shell fuel cell catalysts. Nature Chem. 2, 454–460 (2010).
Koga, K., Ikeshoji, T. & Sugawara, K. Size- and temperature-dependent structural transitions in gold nanoparticles. Phys. Rev. Lett. 92, 115507 (2004).
Moulijn, J. A., van Diepen, A. E. & Kapteijn, F. Catalyst deactivation: Is it predictable? What to do? Appl. Catal. A 212, 3–16 (2001).
Campbell, C. T., Parker, S. C. & Starr, D. E. The effect of size-dependent nanoparticle energetics on catalyst sintering. Science 298, 811–814 (2002).
Qian, L. H., Yan, X. Q., Fujita, T., Inoue, A. & Chen, M. W. Surface enhanced Raman scattering of nanoporous gold: Smaller pore sizes stronger enhancements. Appl. Phys. Lett. 90, 153120 (2007).
Chen, L.Y. et al. Nanoporous PdNi bimetallic catalyst with enhanced electrocatalytic performances for electro-oxidation and oxygen reduction reactions. Adv. Funct. Mater. 21, 4364–4370 (2011).
Zhang, L. et al. Effect of residual silver on surface-enhanced raman scattering of dealloyed nanoporous gold. J. Phys. Chem. C 115, 19583–19587 (2011).
Kresse, G. & Furthmuller, J. Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Acknowledgements
We thank R. E. Dunin-Borkowski and E. A. Stach for fruitful discussions and comments. This work was sponsored by JST-PRESTO, JST-CREST and the Sekisui research fund. J.E. is supported by grant NSF DMR-1003901. We thank the Center for Computational Materials Science, Institute for Materials Research, Tohoku University, for providing us with the Hitachi SR11000 (model K2) supercomputing system and T. Tanji for the gas mixture used in the environmental TEM.
Author information
Authors and Affiliations
Contributions
M.C., T.F. and J.E. conceived and designed the experiments. T.F. and A.H. contributed to the microstructural characterization. P.G. simulated the structures computationally. X.L. and L.Z. fabricated the materials. K.M. modelled the nanoporous structure. Y.I., N.A. and Y.Y. contributed to the evaluation of catalytic performance. T.F. T.T., S.A., Y.Y. and N.T. contributed to environmental TEM observations. M.C., T.F., J.E. and K.M. wrote the paper. All of the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1234 kb)
Supplementary Movie
Supplementary Movie S1 (MOV 4236 kb)
Supplementary Movie
Supplementary Movie S2 (MOV 7278 kb)
Supplementary Movie
Supplementary Movie S3 (MOV 5082 kb)
Supplementary Movie
Supplementary Movie S4 (MOV 6390 kb)
Supplementary Movie
Supplementary Movie S5 (MOV 10476 kb)
Supplementary Movie
Supplementary Movie S6 (MOV 2676 kb)
Rights and permissions
About this article
Cite this article
Fujita, T., Guan, P., McKenna, K. et al. Atomic origins of the high catalytic activity of nanoporous gold. Nature Mater 11, 775–780 (2012). https://doi.org/10.1038/nmat3391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3391
This article is cited by
-
Grain boundary effects in high-temperature liquid-metal dealloying: a multi-phase field study
npj Computational Materials (2023)
-
Structural and optical properties of gold nanosponges revealed via 3D nano-reconstruction and phase-field models
Communications Materials (2023)
-
Generative AI-enabled microstructure design of porous thermal interface materials with desired effective thermal conductivity
Journal of Materials Science (2023)
-
Atomic scale visualizations of low-angle grain boundary mediated plasticity by coupled dislocation climb and glide in nanoporous gold
Nano Research (2023)
-
Uniaxial Compression Behavior of Open Cellular Materials with Spinodal Morphologies Fabricated by Additive Manufacturing
Transactions of the Indian Institute of Metals (2023)