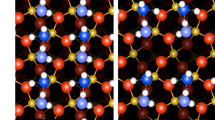
Abstract
Reactions involving minerals and glasses in water are slow and difficult to probe spectroscopically but are fundamental to the performance of oxide materials in green technologies such as automotive thermoelectric power generation1, CO2 capture and storage2 and water-oxidation catalysis3,4; these must be made from geochemically common elements and operate in hydrous environments. Polyoxometalate ions (POMs) have structures similar to condensed oxide phases and can be used as molecular models of the oxide/water interface5. Oxygen atoms in POM exchange isotopes at different rates6,7,8, but, at present, there is no basis for predicting how the coordination environment and metal substitution influences rates and mechanisms. Here we identify low-energy metastable configurations that form from the breaking of weak bonds between metals and underlying highly coordinated oxygen atoms, followed by facile hydroxide, hydronium or water addition. The mediation of oxygen exchange by these stuffed structures suggests a new view of the relationship between structure and reactivity at the oxide/solution interface.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Snyder, G. J. & Toberer, E. S. Complex thermoelectric materials. Nature Mater. 7, 105–114 (2008).
Oelkers, E. H., Gislason, S. R. & Matter, J. Mineral carbonation of CO2 . Elements 4, 333–337 (2008).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Hocking, R. K. et al. Water oxidation catalysts by manganese in a geochemical-like cycle. Nature Chem. 3, 461–466 (2011).
Ohlin, C. A., Villa, E. M., Rustad, J. R. & Casey, W. H. Dissolution of insulating oxide materials at the molecular scale. Nature Mater. 9, 11–19 (2010).
Villa, E. M. et al. Reaction dynamics of the decaniobate ion [HxNb10O28](6−x)− in water. Angew Chem. Int. Edn. 47, 4844–4846 (2008).
Villa, E. M., Ohlin, C. A., Rustad, J. R. & Casey, W. H. Isotope exchange dynamics in isostructural decametalates with profound differences in reactivity. J. Am. Chem. Soc. 131, 16488–16492 (2009).
Villa, E. M., Ohlin, C. A. & Casey, W. H. Oxygen-isotope exchange rates for three isostructural polyoxometalate ions. J. Am. Chem. Soc. 132, 5264–5272 (2010).
Blesa, M. A., Morando, P. J. & Regazzoni, A. E. Chemical Dissolution of Metal Oxides (CRC Press, 1994).
Rustad, J. R., Loring, J. S. & Casey, W. H. Oxygen-exchange pathways in aluminum nanocluster molecules with implications for mineral surfaces. Geochim. Cosmochim. Acta 68, 3011–3017 (2003).
Langford, C. H. & Gray, H. B Ligand Substitution Processes (W. A. Benjamin, 1966).
Hiemstra, T., Van Riemsdijk, W. H. & Bolt, G. H. Multisite proton modeling at the solid/solution interface of (hydr)oxides: A new approach: I. Model description and evaluation of intrinsic reaction constants. J. Colloid Interface Sci. 133, 91–104 (1989).
Henderson, M. A., Joyce, S. A. & Rustad, J. R. Interaction of water with the (1×1) and (2×1) surfaces of α-Fe2O3 (012). Surf. Sci. 417, 66–81 (1998).
Villa, E. M., Ohlin, C. A. & Casey, W. H. Borate accelerates rates of steady oxygen-isotope exchange for polyoxoniobate ions in water. Chem. Eur. J. 16, 8631–8634 (2010).
Connolly, M. L. Analytical molecular surface calculation. J. Appl. Crystallogr. 6, 548–558 (1983).
Handy, N. C. & Cohen, A. J. Left-right correlation energy. Mol. Phys. 99, 403–412 (2001).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split-valence, triple-zeta valence, and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Klamt, A. & Shuurmann, G. COSMO—A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2, 799–805 (1993).
Acknowledgements
Support for this research was funded by the US Department of Energy Office of Basic Energy Science via grant DE-FG02-05ER15693 and National Science Foundation via EAR grant 0814242.
Author information
Authors and Affiliations
Contributions
J.R.R. designed and performed the calculations, W.H.C and J.R.R. interpreted the experiments in the context of the calculations, J.R.R. and W.H.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 522 kb)
Rights and permissions
About this article
Cite this article
Rustad, J., Casey, W. Metastable structures and isotope exchange reactions in polyoxometalate ions provide a molecular view of oxide dissolution. Nature Mater 11, 223–226 (2012). https://doi.org/10.1038/nmat3203
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3203
This article is cited by
-
Rapid oxygen exchange between hematite and water vapor
Nature Communications (2021)
-
Linear topology in amorphous metal oxide electrochromic networks obtained via low-temperature solution processing
Nature Materials (2016)
-
Stuffed structures
Nature Materials (2012)