Abstract
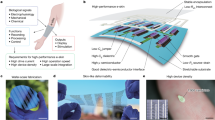
Developing advanced surgical tools for minimally invasive procedures represents an activity of central importance to improving human health. A key challenge is in establishing biocompatible interfaces between the classes of semiconductor device and sensor technologies that might be most useful in this context and the soft, curvilinear surfaces of the body. This paper describes a solution based on materials that integrate directly with the thin elastic membranes of otherwise conventional balloon catheters, to provide diverse, multimodal functionality suitable for clinical use. As examples, we present sensors for measuring temperature, flow, tactile, optical and electrophysiological data, together with radiofrequency electrodes for controlled, local ablation of tissue. Use of such ‘instrumented’ balloon catheters in live animal models illustrates their operation, as well as their specific utility in cardiac ablation therapy. The same concepts can be applied to other substrates of interest, such as surgical gloves.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mueller, R. & Sanborn, T. The history of interventional cardiology. Am. Heart J. 129, 146–172 (1995).
Myler, R. & Stertzer, S. Textbook of Interventional Cardiology Ch. 21 (WB Saunders, 1993).
Rich, S., Dodin, E. & McLaughlin, V. V. Usefulness of atrial septostomy as a treatment for primary pulmonary hypertension and guidelines for its application. Am. J. Cardiol. 80, 369–371 (1997).
Rothman, A. et al. Graded balloon dilation atrial septostomy as a bridge to lung transplantation in pulmonary hypertension. Am. Heart J. 125, 1763–1766 (1993).
Aliot, E. M. et al. Expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up, a report of the heart rhythm society (HRS) task force on catheter and surgical ablation of atrial fibrillation. Europace 9, 335–379 (2007).
Calkins, H. et al. Treatment of atrial fibrillation with antiarrhythmic drugs for radio frequency ablation: Two systematic literature reviews and meta-analyses. Circ. Arrhythmia Electrophysiol. 2, 349–361 (2009).
Dewire, J. & Calkins, H. State-of-the-art and emerging technologies for atrial fibrillation ablation. Nature Rev. Cardiol. 7, 129–138 (2010).
Chun, K. R. et al. The ‘single big cryoballoon’ technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: A prospective observational single centre study. Euro. Heart J. 30, 699–709 (2009).
Chun, K. R. et al. Cryoballoon pulmonary vein isolation with real time recordings from the pulmonary veins. J. Cardiovasc. Electrophysiol. 30, 699–709 (2009).
Satake, S. et al. Usefulness of a new radiofrequency thermal balloon catheter for pulmonary vein isolation: A new device for treatment of atrial fibrillation. J. Cardiovasc. Electrophysiol. 14, 609–615 (2003).
Schmidt, B. et al. Pulmonary vein isolation by high intensity focused ultrasound: First in-man study with a steerable balloon catheter. Heart Rhythm. 4, 575–584 (2007).
Reddy, V. Y. et al. Visually-guided balloon catheter ablation of atrial fibrillation: Experimental feasibility and first in-human multicenter clinical outcome. Circulation 120, 12–20 (2009).
Reddy, V. Y. et al. Balloon catheter ablation to treat paroxysmal atrial fibrillation: What is the level of venous isolation? Heart Rhythm. 5, 353–360 (2008).
Mansour, M. et al. Initial experience with the Mesh catheter for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 5, 1510–1516 (2008).
Meissner, A. et al. First experiences for pulmonary vein isolation with the high density mesh ablator (HDMA): A novel mesh electrode catheter for both mapping and radiofrequency delivery in a single unit. J. Cardiovasc. Electrophysiol. 20, 359–366 (2009).
Lau, M. et al. A theoretical and experimental analysis of radiofrequency ablation with a multielectrode, phased, duty-cycled system. PACE 33, 1089–1100 (2010).
Di Biase, L. et al. Relationship between catheter forces, lesions, characteristics, popping and char formation: Experience with robotic navigation system. J. Cardiovasc. Electrophysiol. 20, 436–440 (2009).
Kim, D-H. et al. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl Acad. Sci. USA 105, 18675–18680 (2008).
Viventi, J. et al. A Conformal, bio-interfaced class of silicon electronics for mapping cardiac electrophysiology. Sci. Translational Med. 2, 24ra22 (2010).
Kim, R-H. et al. Waterproof AlInGaP optoelectronics on stretchable substrates with applications in biomedicine and robotics. Nature Mater. 9, 929–937 (2010).
Mathura, A. B., Collinswortha, A. M., Reicherta, W. M., Krausb, W. E. & Truskey, G. A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomechanics. 34, 1545–1553 (2001).
Lee, M. H. & Nicholls, H. R. Tactile sensing for mechatronics—a state of the art survey. Mechatronics 9, 1–31 (1999).
Dargahi, J., Parameswaran, M. & Payandeh, S. A micromachined piezoelectric tactile sensor for an endoscopic grasper—theory, fabrication and experiments. J. Microelectromech. Syst. 9, 329–335 (2000).
Engel, J., Chen, J. & Liu, C. Development of polyimide flexible tactile sensor skin. J. Micromech. Microeng. 13, 359–366 (2003).
Someya, T. et al. A large-area, flexible pressure sensor matrix with organic field-effect transistors for artificial skin applications. Proc. Natl Acad. Sci. USA 101, 9966–9970 (2004).
Takei, K. et al. Nanowire active-matrix circuitry for low-voltage macroscale artificial skin. Nature Mater. 9, 821–826 (2010).
Kim, D-H. et al. Optimized structural designs for stretchable silicon integrated circuits. Small 5, 2841–2847 (2009).
Sun, J-Y. et al. Inorganic islands on a highly stretchable polyimide substrate. J. Mater. Res. 24, 3338–3342 (2009).
Berjano, E. J. Theoretical modelling for radiofrequency ablation: State-of-the-art and challenges for the future. Biomed. Eng. Online 5, 24 (2006).
Plonsey, R. & Heppner, D. B. Considerations of quasi-stationarity in electrophysiological systems. Bul. Math. Biophys. 29, 657–664 (1967).
Huang, L. & Liu, L. Simultaneous determination of thermal conductivity and thermal diffusivity of food and agricultural materials using a transient plane-source method. J. Food Eng. 95, 179–185 (2009).
Zell, M., Lyng, J. G., Cronin, D. A. & Morgan, D. J. Ohmic heating of meats: Electrical conductivities of whole meats and processed meat ingredients. Meat. Sci. 83, 563–570 (2009).
Erez, A. & Shitzer, A. Controlled destruction and temperature distribution in biological tissues subjected to monoactive electrocoagulation. J. Biomed. Eng. 102, 42–49 (1980).
Jiang, G. & Zhou, D. D. in Implantable Neural Prostheses 2, Biological and Medical Physics (eds Zhou, D. D. & Greenbaum, E.) Ch. 2, 27–61 (Springer, 2010).
Machi, E. et al. High density epicardial mapping during current injection and ventricular activation in rat hearts. Am. J. Heart Circ. Physiol. 275, H1886–H1897 (1998).
Bélanger, M. C. & Marois, Y. Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review. J. Biomed. Mater. Res. 58, 467–477 (2001).
Richardson, R. R. Jr, Miller, J. A. & Reichert, W. M. Polyimides as biomaterials: Preliminary biocompatibility testing. Biomaterials 14, 627–635 (1993).
Wit, A. L. & Janse, M. J. Experimental models of ventricular tachycardia and fibrillation caused by ischemia and infarction. Circulation 85, I-32-I-42 (1992).
Shin, M. K. et al. Elastomeric conductive composites based on carbon nanotube forests. Adv. Mater. 22, 2663–2667 (2010).
Acknowledgements
We thank K. Dowling for help in high-resolution imaging and analysis of devices. We thank B. Dehdashti and members of the Sarver Heart Center for help in preparing the animals for in vivo studies. We thank S. Laferriere and the Massachusetts General Hospital Electrophysiology Laboratory for help with X-ray imaging in animals. This material is based on work supported by the National Science Foundation under grant DMI-0328162 and the US Department of Energy, Division of Materials Sciences, under award DE-FG02-07ER46471, through the Materials Research Laboratory and Center for Microanalysis of Materials (DE-FG02-07ER46453) at the University of Illinois at Urbana-Champaign. N.L. acknowledges support from a Beckman Institute postdoctoral fellowship. J.A.R. acknowledges a National Security Science and Engineering Faculty Fellowship.
Author information
Authors and Affiliations
Contributions
D-H.K., N.L., R.G. and J.A.R. designed the experiments. D-H.K., N.L., R.G., Y-S.K., S.P.L., L.X., J.W., R-H.K., J.S., Z.L., B.D.G., B.E., M.J.S., S.H., J.V., J.D.M., S-M.W., Y.H., B.L. and J.A.R. carried out experiments and analysis. D-H.K., N.L., R.G., M.M., M.J.S., J.S., Y.H. and J.A.R. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare competing financial interests: R.G., S.P.L. and B.dG. are employees with a company (MC10, Inc.) that is developing catheter-based devices for cardiac therapy. J.A.R. is a founder of this company; he and M.S. serve as advisors.
Supplementary information
Supplementary Information
(PDF 2492 kb)
Rights and permissions
About this article
Cite this article
Kim, DH., Lu, N., Ghaffari, R. et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nature Mater 10, 316–323 (2011). https://doi.org/10.1038/nmat2971
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2971
This article is cited by
-
Soft bioelectronics for the management of cardiovascular diseases
Nature Reviews Bioengineering (2023)
-
Adhesive bioelectronics for sutureless epicardial interfacing
Nature Electronics (2023)
-
Temperature sensing properties of a flexible organic single-crystal field-effect transistor
Journal of Materials Science: Materials in Electronics (2023)
-
Finite element analysis of the interaction between high-compliant balloon catheters and non-cylindrical vessel structures: towards tactile sensing balloon catheters
Biomechanics and Modeling in Mechanobiology (2023)
-
Minimally-invasive cardiac surgery: a bibliometric analysis of impact and force to identify key and facilitating advanced training
Journal of Cardiothoracic Surgery (2022)