Abstract
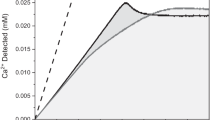
Unravelling the processes of calcium phosphate formation1,2,3,4 is important in our understanding of both bone and tooth formation5,6,7, and also of pathological mineralization, for example in cardiovascular disease8,9,10. Serum is a metastable solution from which calcium phosphate precipitates in the presence of calcifiable templates such as collagen, elastin and cell debris11,12. A pathological deficiency of inhibitors leads to the uncontrolled deposition of calcium phosphate. In bone and teeth the formation of apatite crystals is preceded by an amorphous calcium phosphate (ACP) precursor phase13,14. ACP formation is thought to proceed through prenucleation clusters—stable clusters that are present in solution already before nucleation—as was recently demonstrated for CaCO3 (refs 1516). However, the role of such nanometre-sized clusters as building blocks2 for ACP has been debated for many years. Here we demonstrate that the surface-induced formation of apatite from simulated body fluid17,18 starts with the aggregation of prenucleation clusters leading to the nucleation of ACP before the development of oriented apatite crystals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Brecevic, L. J. & Furedi-Milhofer, H. Precipitation of calcium phosphates from electrolyte solutions. II. The formation and transformation of precipitates. Calcif. Tissue Res. 10, 82–90 (1972).
Posner, A. S. & Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 8, 273–281 (1975).
Onuma, K. & Ito, A. Cluster growth model for hydroxyapatite. Chem. Mater. 10, 3346–3351 (1998).
Müller, F. A., Müller, L., Caillard, D. & Conforto, E. Preferred growth orientation of biomimetic apatite crystals. J. Cryst. Growth 304, 464–471 (2007).
Brown, W. E. & Chow, L. C. Chemical properties of bone mineral. Annu. Rev. Mater. Sci. 6, 213–236 (1976).
Smith, C. E. Cellular and chemical events during enamel maturation. Crit. Rev. Oral Biol. Med. 9, 128–161 (1998).
Olszta, M. J. et al. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 58, 77–116 (2007).
Luo, G. et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386, 78–81 (1997).
Hui, M., Li, S. Q., Holmyard, D. & Cheng, P. T. Stable transfection of nonosteogenic cell lines with tissue nonspecific alkaline phosphatase enhances mineral deposition both in the presence and absence of β-glycerophosphate: Possible role for alkaline phosphatase in pathological mineralisation. Calcif. Tissue Int. 60, 467–472 (1997).
Dorozhkin, S. V. & Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 41, 3130–3146 (2002).
Urry, D. W. Molecular basis for vascular calcification. Perspect. Biol. Med. 17, 68–84 (1974).
Westenfeld, R. et al. Fetuin-A protects against atherosclerotic calcification in CKD. J. Am. Soc. Nephrol. 20, 1264–1274 (2009).
Beniash, E., Metzler, R. A., Lam, R. S. K. & Gilbert, P. U. P. A. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 166, 133–143 (2009).
Mahamid, J. et al. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl Acad. Sci. USA 107, 6316–6321 (2010).
Gebauer, D., Volkel, A. & Colfen, H. Stable prenucleation calcium carbonate clusters. Science 322, 1819–1822 (2008).
Pouget, E. M. et al. The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 323, 1455–1458 (2009).
Ayako, O. et al. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. 65A, 188–195 (2003).
Muller, L. & Muller, F. A. Preparation of SBF with different HCO3 content and its influence on the composition of biomimetic apatites. Acta Biomater. 2, 181–189 (2006).
Rey, C., Combes, C., Drouet, C. & Glimcher, M. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 20, 1013–1021 (2009).
Auer, S. & Frenkel, D. Prediction of absolute crystal-nucleation rate in hard-sphere colloids. Nature 409, 1020–1023 (2001).
Wang, L. & Nancollas, G. H. Pathways to biomineralization and biodemineralization of calcium phosphates: The thermodynamic and kinetic controls. Dalton Trans. 2665–2672 (2009).
Erdemir, D., Lee, A. Y. & Myerson, A. S. Nucleation of crystals from solution: Classical and two-step models. Acc. Chem. Res. 42, 621–629 (2009).
Pichon, B. P., Bomans, P. H. H., Frederik, P. M. & Sommerdijk, N. A. J. M. A quasi-time-resolved cryoTEM study of the nucleation of CaCO3 under Langmuir monolayers. J. Am. Chem. Soc. 130, 4034–4040 (2008).
Legeros, R. Z., Trautz, O. R., Legeros, J. P., Klein, E. & Shirra, W. P. Apatite crystallites: Effects of carbonate on morphology. Science 155, 1409–1411 (1967).
Ivanova, T. I., Frank-Kamenetskaya, O. V., Kol’tsov, A. B. & Ugolkov, V. L. Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J. Solid State Chem. 160, 340–349 (2001).
Sato, K., Kogure, T., Kumagai, Y. & Tanaka, J. Crystal orientation of hydroxyapatite induced by ordered carboxylic groups. J. Colloid Interface Sci. 240, 133–138 (2001).
DiMasi, E., Olszta, M. J., Patel, V. M. & Gower, L. B. When is template directed mineralization really template directed? Cryst. Eng. Comm. 5, 346–350 (2003).
Xilin, Y. & Malcolm, J. S. Biological calcium phosphates and Posner’s cluster. J. Chem. Phys. 118, 3717–3723 (2003).
Termine, J. D. & Posner, A. S. Calcium phosphate formation in vitro: I. Factors affecting initial phase separation. Arch. Biochem. Biophys. 140, 307–317 (1970).
Nudelman, F. et al. Collagen can directly control the formation of bone apatite in the presence of a calcium-ion binding polymer in solution. Nature Mater. published online doi:10.1038/NMAT2875 (24 October 2010).
Tsuji, T., Onuma, K., Yamamoto, A., Iijima, M. & Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif-programmed artificial proteins. Proc. Natl Acad. Sci. USA 105, 16866–16870 (2008).
Crane, N. J., Popescu, V., Morris, M. D., Steenhuis, P. & Ignelzi, J. M. A. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 39, 434–442 (2006).
Bohner, M. & Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 30, 2175–2179 (2009).
Mitchell, D. R. G. DiffTools: Electron diffraction software tools for digitalmicrograph. Microsc. Res. Tech. 71, 588–593 (2008).
Acknowledgements
Supported by the European Community (project code NMP4-CT-2006-033277) and the Netherlands Organization for Scientific Research (NWO). We are grateful to F. Nudelman for discussions and experimental assistance. We thank E. M. Pouget for her help in experimental design, F. L. Boogaard and J. J. van Roosmalen for their contribution to the three-dimensional reconstructions of the tomograms, M.M.R.M. Hendrix for his help with X-ray diffraction and P. T. K. Chin for providing the CdSe nanorods.
Author information
Authors and Affiliations
Contributions
A.D. carried out all experiments and cowrote the manuscript. P.H.H.B. and P.M.F. provided support with the cryoTEM. F.A.M. and J.W. provided SBF. G.W. and N.A.J.M.S. supervised the project and N.A.J.M.S. cowrote the manuscript. All authors discussed the results and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. S1-S8, Table S1-Table S2
Supplementary Information (PDF 1352 kb)
Supplementary Information
Supplementary Information Movie 1 (MOV 3474 kb)
Supplementary Information
Supplementary Information Movie 2 (MOV 3803 kb)
Supplementary Information
Supplementary Information Movie 3 (MOV 3053 kb)
Supplementary Information
Supplementary Information Movie 4 (MOV 3292 kb)
Supplementary Information
Supplementary Information Movie 5 (MOV 2580 kb)
Supplementary Information
Supplementary Information Movie 6 (MOV 3204 kb)
Rights and permissions
About this article
Cite this article
Dey, A., Bomans, P., Müller, F. et al. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nature Mater 9, 1010–1014 (2010). https://doi.org/10.1038/nmat2900
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2900
This article is cited by
-
Redefined ion association constants have consequences for calcium phosphate nucleation and biomineralization
Nature Communications (2024)
-
Inorganic ionic polymerization: From biomineralization to materials manufacturing
Nano Research (2024)
-
Development of peptide–inorganic hybrid materials based on biomineralization and their functional design based on structural controls
Polymer Journal (2023)
-
Real-time evolution of up-conversion nanocrystals from tailored metastable intermediates
Nano Research (2023)
-
Biomineralization of bone tissue: calcium phosphate-based inorganics in collagen fibrillar organic matrices
Biomaterials Research (2022)