Abstract
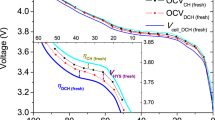
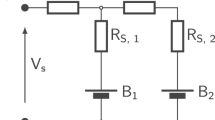
Lithium batteries are considered the key storage devices for most emerging green technologies such as wind and solar technologies or hybrid and plug-in electric vehicles. Despite the tremendous recent advances in battery research, surprisingly, several fundamental issues of increasing practical importance have not been adequately tackled. One such issue concerns the energy efficiency. Generally, charging of 1010–1017 electrode particles constituting a modern battery electrode proceeds at (much) higher voltages than discharging. Most importantly, the hysteresis between the charge and discharge voltage seems not to disappear as the charging/discharging current vanishes. Herein we present, for the first time, a general explanation of the occurrence of inherent hysteretic behaviour in insertion storage systems containing multiple particles. In a broader sense, the model also predicts the existence of apparent equilibria in battery electrodes, the sequential particle-by-particle charging/discharging mechanism and the disappearance of two-phase behaviour at special experimental conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Boyanov, S. et al. P-redox mechanism at the origin of the high lithium storage in NiP2-based batteries. Chem. Mater. 21, 298–308 (2009).
Doe, R. E., Persson, K. A., Meng, Y. S. & Ceder, G. First-principles investigation of the Li–Fe–F phase diagram and equilibrium and nonequilibrium conversion reactions of iron fluorides with lithium. Chem. Mater. 20, 5274–5283 (2008).
Ogasawara, T., Débart, A., Holzapfel, M., Novák, P. & Bruce, P. G. Rechargeable Li2O2 electrode for lithium batteries. J. Am. Chem. Soc. 128, 1390–1393 (2006).
Yamada, A. et al. Room-temperature miscibility gap in LixFePO4 . Nature Mater. 5, 357–360 (2006).
Dreyer, W., Guhlke, C. & Huth, R. The behaviour of a many particle cathode in a lithium-ion battery, WIAS Preprint No. 1423 (2009).
Han, B. C., Van der Ven, A., Morgan, D. & Ceder, G. Electrochemical modeling of intercalation processes with phase field models. Electrochim. Acta 49, 4691–4699 (2004).
Amin, R., Balaya, P. & Maier, J. Anisotropy of electronic and ionic transport in LiFePO4 single crystals. Electrochem. Solid State Lett. 10, A13–A16 (2007).
Kang, B. & Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009).
Srinivasan, V. & Newman, J. Discharge model for the lithium iron–phosphate electrode. J. Electrochem. Soc. 151, A1517–A1529 (2004).
Srinivasan, V. & Newman, J. Existence of path-dependence in the LiFePO4 electrode. Electrochem. Solid State Lett. 9, A110–A114 (2006).
Delmas, C., Maccario, M., Croguennec, L., Le Cras, F. & Weill, F. Lithium deintercalation in LiFePO4 nanoparticles via a domino-cascade model. Nature Mater. 7, 665–671 (2008).
Wagemaker, M., Borghols, W. J. H. & Mulder, F. M. Large impact of particle size on insertion reaction. A case for anatase LixTiO2 . J. Am. Chem. Soc. 129, 4323–4327 (2007).
Hainovsky, N. & Maier, J. Simple phenomenological approach to premelting and sublattice melting in Frenkel disordered ionic crystals. Phys. Rev. B 51, 15789–15797 (1995).
Müller, I. & Strehlow, P. Rubber and Rubber Balloons (Lecture Notes in Physics. Vol. 637, Springer, 2004).
Gibot, P. et al. Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4 . Nature Mater. 7, 741–747 (2008).
Dominko, R., Conte, R. D., Hanzel, D., Gaberscek, M. & Jamnik, J. Impact of synthesis conditions on the structure and performance of Li2FeSiO4 . J. Power Sources 178, 842–847 (2008).
Erjavec, B. et al. Tailoring nanostructured TiO2 for high power Li-ion batteries. J. Power Sources 189, 869–874 (2009).
Dominko, R. et al. Wired porous cathode materials: A novel concept for synthesis of LiFePO4 . Chem. Mater. 19, 2960–2969 (2007).
Acknowledgements
The main idea for this work was developed within the former ALISTORE Network of Excellence (now ALISTORE-ERI). The work has been partly supported by the Slovenian Research Agency (Grant No. P2-0148). Another part of the work was carried out as part of Project C26 ‘Storage of Hydrogen in Hydrides’ of the DFG research centre MATHEON, Berlin.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 5125 kb)
Rights and permissions
About this article
Cite this article
Dreyer, W., Jamnik, J., Guhlke, C. et al. The thermodynamic origin of hysteresis in insertion batteries. Nature Mater 9, 448–453 (2010). https://doi.org/10.1038/nmat2730
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2730
This article is cited by
-
Accelerating the transition to cobalt-free batteries: a hybrid model for LiFePO4/graphite chemistry
npj Computational Materials (2024)
-
The fluidic memristor as a collective phenomenon in elastohydrodynamic networks
Nature Communications (2024)
-
Progress in the prognosis of battery degradation and estimation of battery states
Science China Materials (2024)
-
On the Impact of Mechanics on Electrochemistry of Lithium-Ion Battery Anodes
JOM (2024)
-
Thermodynamics of multi-sublattice battery active materials: from an extended regular solution theory to a phase-field model of LiMnyFe1-yPO4
npj Computational Materials (2023)