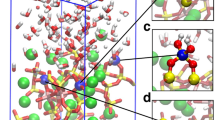
Abstract
Our understanding of mineral and glass dissolution has advanced from simple thermodynamic treatments to models that emphasize adsorbate structures. This evolution was driven by the idea that the best understanding is built at the molecular level. Now, it is clear that the molecular questions cannot be answered uniquely with dissolution experiments. At the surface it is unclear which functional groups are present, how they are arranged, and how they interact with each other and with solutes as the key bonds are activated. An alternative approach has developed whereby reactions are studied with nanometre-sized aqueous oxide ions that serve as models for the more complicated oxide interface. For these ions, establishing the structure is not a research problem in itself, and bond ruptures and dissociations can be followed with much confidence. We review the field from bulk-dissolution kinetics to the new isotope-exchange experiments in large oxide ions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
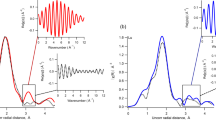
References
Blesa, M. A., Morando, P. J. & Regazzoni, A. E. Chemical Dissolution of Metal Oxides 432 (CRC Press, 1993).
Orme, C. A. et al. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 411, 775–779 (2001).
Cailleteau, C. et al. Insight into silicate-glass corrosion mechanisms. Nature Mater. 7, 978–983 (2008).
Eisenbarth, S. C., Colegio, O. R., O'Connor, W., Sutterwala, F. S. & Flavell, R. A. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 (2008).
Allouche, L., Gerardin, C., Loiseau, T., Ferey, G. & Taulelle, F. Al30: a giant aluminum polycation. Angew. Chem., Int. Ed. 39, 511–514 (2000).
Yanina, S. V. & Rosso, K. M. Linked reactivity at mineral-water interfaces through bulk crystal conduction. Science 320, 218–222 (2008).
Cesar, I., Kay, A., Gonzalez Martinez, J. A. & Grätzel, M. Translucent thin film Fe2O3 photoanodes for efficient water splitting by sunlight: nanostructure-directing effect of Si-doping. J. Am. Chem. Soc. 128, 4582–4583 (2006).
Furrer, G., Phillips, B. L., Ulrich, K. U., Pothig, R. & Casey, W. H. The origin of aluminum flocs in polluted streams. Science 297, 2245–2247 (2002).
Catalano, J. G., Fenter, P. & Park, C. Water ordering and surface relaxations at the haematite (110)-water interface. Geochim. Cosmochim. Acta 73, 2242–2251 (2009).
Zhang, Z. et al. Structure of rutile TiO2 (110) in water and 1 molal Rb+ at pH 12: Inter-relationship among surface charge, interfacial hydration structure, and substrate structural displacements. Surf. Sci. 601, 1129–1143 (2007).
Fenter, P. A., Rivers, M. L., Sturchio, N. C. & Sutton, S. R. (eds) Applications of Synchrotron Radiation in Low-Temperature Geochemistry and Environmental Sciences Vol. 49, 579 (Mineralogical Soc. America, 2002).
Hamilton, J. P., Pantano, C. G. & Brantley, S. L. Dissolution of albite glass and crystal. Geochim. Cosmochim. Acta 64, 2603–2615 (2000).
Casey, W. H. & Westrich, H. R. Control of dissolution rates of orthosilicate minerals by divalent metal-oxygen bonds. Nature 355, 157–159 (1992).
Bunker, B. C. Molecular mechanisms for corrosion of silica and silicate glasses. J. Non-Cryst. Solids 179, 300–308 (1994).
Bunker, B. C., Arnold, G. W., Beauchamp, E. K. & Day, D. E. Mechanisms for alkali leaching in mixed sodium-potassium silicate glasses. J. Non-Cryst. Solids 58, 295–322 (1983).
Casey, W. H., Westrich, H. R., Massis, T., Banfield, J. F. & Arnold, G. W. The surface of labradorite feldspar after acid hydrolysis. Chem. Geol. 7 8, 205–18 (1989).
Casey, W. H., Westrich, H. R., Banfield, J. F., Ferruzzi, G. & Arnold, G. W. Leaching and reconstruction at the surfaces of dissolving chain-silicate minerals. Nature 366, 253–256 (1993).
Asta, M. P., Cama, J., Soler, J. M., Arvidson, R. S. & Luttge, A. Interferometric study of pyrite surface reactivity in acidic conditions. Am. Mineral. 93, 508–519 (2008).
Green, E. & Luttge, A. Incongruent dissolution of wollastonite measured with vertical scanning interferometry. Am. Mineral. 91, 430–434 (2006).
Berner, R. A. & Holdren, G. R. Jr Mechanism of feldspar weathering. II. Observations of feldspars from soils. Geochim. Cosmochim. Acta 43, 1173–1186 (1979).
Berner, R. A. Rate control of mineral dissolution under earth surface conditions. Am. J. Sci. 278, 1235–1252 (1978).
Maher, K., Steefel, C. I., White, A. F. & Stonestrom, D. A. The role of reaction affinity and secondary minerals in regulating chemical weathering rates at the Santa Cruz Soil Chronosequence, California. Geochim. Cosmochim. Acta. 73, 2804–2831 (2009).
Michel, F. M. et al. The structure of ferrihydrite, a nanocrystalline material. Science 316, 1726–1729 (2007).
Casey, W. H. Dynamics and durability. Nature Mater. 7, 930–932 (2008).
Boudart, M. & Djega-Mariadasson, G. in Kinetics of Heterogeneous Catalytic Reactions (eds Brewer, L. & Prausnitz, J. M.) Ch. 3, 9–11 (Princeton Univ. Press, 1984).
Dove, P. M., Han, N. & De Yoreo, J. J. Mechanisms of classical crystal growth theory explain quartz and silicate dissolution behaviour. Proc. Natl Acad. Sci. USA 102, 15357–15362 (2005).
Burton, W. K., Cabrera, N. & Frank, F. C. Dislocations in crystal growth. Nature 163, 398–399 (1949).
Thomas, T. N., Land, T. A., Casey, W. H. & DeYoreo, J. J. Emergence of supersteps on KH2PO4 crystal surfaces. Phys. Rev. Lett. 92, 216103 (2004).
Furrer, G. & Stumm, W. The coordination chemistry of weathering: I. Dissolution kinetics of alumina and beryllium oxide. Geochim. Cosmochim. Acta 50, 1847–1860 (1986).
Zinder, B., Furrer, G. & Stumm, W. The coordination chemistry of weathering: II. Dissolution of iron(III) oxides. Geochim. Cosmochim. Acta 50, 1861–1869 (1986).
Koch, G. Kinetics and mechanism of the solution of beryllium oxide in acids. Ber. Bunsenges. Phys. Chem. 69, 141–145 (1965).
Loring, J. S., Sandstroem, M. H., Noren, K. & Persson, P. Rethinking arsenate coordination at the surface of goethite. Chem. Eur. J. 15, 5063–5072 (2009).
Boily, J.-F. & Felmy, A. R. On the protonation of oxo- and hydroxo-groups of the goethite (α-FeOOH) surface: A FTIR spectroscopic investigation of surface O–H stretching vibrations. Geochim. Cosmochim. Acta 72, 3338–3357 (2008).
Rochester, C. H. & Topham, S. A. Infrared study of the surface hydroxyl groups on goethite. J. Chem. Soc. Faraday Trans I 75, 591–602 (1979).
Bargar, J. R., Kubicki, J. D., Reitmeyer, R. & Davis, J. A. ATR-FTIR spectroscopic characterization of coexisting carbonate surface complexes on haematite. Geochim. Cosmochim. Acta 69, 1527–1542 (2005).
Sverjensky, D. A. Standard states for the activities of mineral surface sites and species. Geochim. Cosmochim. Acta 67, 17–28 (2003).
van Riemsdijk, W. H. & Hiemstra, T. The CD-MUSIC model as a framework for interpreting ion adsorption on metal (hydr)oxide surfaces. Interf. Sci. Tech. 11, 251–268 (2006).
Lasaga, A. C. Kinetic Theory in the Earth Sciences 728 (Princeton Univ. Press, 1998).
Gibbs, G. V. Molecules as models for bonding in silicates. Am. Mineral. 67, 421–450 (1982).
Rotzinger, F. P. Performance of molecular orbital methods and density functional theory in the computation of geometries and energies of metal aqua ions. J. Phys. Chem. B 109, 1510–1527 (2005).
Evans, R. J., Rustad, J. R. & Casey, W. H. Calculating geochemical reaction pathways - Exploration of the inner-sphere water exchange mechanism in Al(H2O)63+(aq) + nH2O with ab initio calculations and molecular dynamics. J. Phys. Chem. A 112, 4125–4140 (2008).
Brown, G. E. Jr et al. Metal oxide surfaces and their interactions with aqueous solutions and microbial organisms. Chem. Rev. 99, 77–174 (1999).
Hochella, M. F. Jr in Mineral-Water Interface Geochemistry Vol. 23 (eds Hochella, M. F. Jr & White, A. F.) 87–132 (Mineralogical Soc. America, 1991).
Stirniman, M. J., Huang, C., Smith, R. S., Joyce, S. A. & Kay, B. D. The adsorption and desorption of water on single crystal MgO(100): The role of surface defects. J. Chem. Phys. 105, 1295–1298 (1996).
Guenard, P., Renaud, G., Barbier, A. & Gautier-Soyer, M. Determination of the α-Al2O3(0001) Surface relaxation and termination by measurements of crystal truncation rods. Surf. Rev. Lett. 5, 321–324 (1998).
Stanka, B., Hebenstreit, W., Diebold, U. & Chambers, S. A. Surface reconstruction of Fe3O4(001). Surf. Sci. 448, 49–63 (2000).
Joseph, Y., Ranke, W. & Weiss, W. Water on FeO(111) and Fe3O4(111): Adsorption behaviour on different surface terminations. J. Phys. Chem. B 104, 3224–3236 (2000).
Thevuthasan, S. et al. Surface structure of MBE-grown α-Fe2O3(0001) by intermediate-energy X-ray photoelectron diffraction. Surf. Sci. 425, 276–286 (1999).
Henderson, M. A., Joyce, S. A. & Rustad, J. R. Interaction of water with the (1×1) and (2×1) surfaces of α-Fe2O3(012). Surf. Sci. 417, 66–81 (1998).
Henderson, M. A. An HREELS and TPD study of water on TiO2(110): The extent of molecular versus dissociative adsorption. Surf. Sci. 355, 151–166 (1996).
Wang, Y., Nguyen, H. N. & Truong, T. N. Mechanisms of and effect of coadsorption on water dissociation on an oxygen vacancy of the MgO(100) surface. Chem. Eur. J. 12, 5859–5867 (2006).
Kowalski, P. M., Meyer, B. & Marx, D. Composition, structure, and stability of the rutile TiO2(110) surface: Oxygen depletion, hydroxylation, hydrogen migration, and water adsorption. Phys. Rev. B. 79, 115410–115411 (2009).
Wang, X. G. et al. The haematite (α-Fe2O3) (0001) surface: Evidence for domains of distinct chemistry. Phys. Rev. Lett. 81, 1038–1041 (1998).
Brudvig, G. W. Water oxidation chemistry of photosystem II. Phil. Trans. R. Soc. B 363, 1211–1219 (2008).
Xu, Y., Feng, L., Jeffrey Philip, D., Shi, Y. & Morel Francois, M. M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 452, 56–61 (2008).
Casey, W. H. & Swaddle, T. W. Why small? The use of small inorganic clusters to understand mineral surface and dissolution reactions in geochemistry. Rev. Geophys. 41, 4/1–4/20 (2003).
Powell, A. K. Polyiron oxides, oxyhydroxides and hydroxides as models for biomineralization processes. Struct. Bond. 88, 1–38 (1997).
Richens, D. T. Ligand substitution reactions at inorganic centers. Chem. Rev. 105, 1961–2002 (2005).
Rotzinger, F. P. Treatment of substitution and rearrangement mechanisms of transition metal complexes with quantum chemical methods. Chem. Rev. 105, 2003–2037 (2005).
Erras-Hanauer, H., Clark, T. & van Eldik, R. Molecular orbital and DFT studies on water exchange mechanisms of metal ions. Coord. Chem. Rev. 238–239, 233–253 (2003).
Wang, J., Rustad, J. R. & Casey, W. H. Calculation of water-exchange rates on aqueous polynuclear clusters and at oxide-water interfaces. Inorg. Chem. 46, 2962–2964 (2007).
Stack, A. G. & Rustad, J. R. Structure and dynamics of water on aqueous barium ion and the {001} barite surface. J. Phys. Chem. C 111, 16387–16391 (2007).
Catalano, J. G., Park, C., Zhang, Z. & Fenter, P. Termination and water adsorption at the α-Al2O3 (012)-aqueous solution interface. Langmuir 22, 4668–4673 (2006).
Catalano, J. G., Fenter, P. & Park, C. Interfacial water structure on the (012) surface of haematite: Ordering and reactivity in comparison with corundum. Geochim. Cosmochim. Acta 71, 5313–5324 (2007).
Nangia, S. & Garrison, B. J. Reaction rates and dissolution mechanisms of quartz as a function of pH. J. Phys. Chem. A 112, 2027–2033 (2008).
Xiao, Y. & Lasaga, A. C. Ab initio quantum mechanical studies of the kinetics and mechanisms of quartz dissolution: OH− catalysis. Geochim. Cosmochim. Acta 60, 2283–2295 (1996).
Xiao, Y. & Lasaga, A. C. Ab initio quantum mechanical studies of the kinetics and mechanisms of silicate dissolution: H+(H3O+) catalysis. Geochim. Cosmochim. Acta 58, 5379–5400 (1994).
Kubicki, J. D., Blake, G. A. & Apitz, S. E. Ab initio calculations on aluminosilicate Q3 species: implications for atomic structures of mineral surfaces and dissolution mechanisms of feldspars. Am. Mineral. 81, 789–799 (1996).
Criscenti, L. J., Kubicki, J. D. & Brantley, S. L. Silicate glass and mineral dissolution: Calculated reaction paths and activation energies for hydrolysis of a Q3 Si by H3O+ using ab initio methods. J. Phys. Chem. A 110, 198–206 (2006).
Bradley, S. M., Kydd, R. A. & Howe, R. F. The structure of Al gels formed through the base hydrolysis of Al3+ aqueous solutions. J. Colloid Interface Sci. 159, 405–412 (1993).
Bradley, S. M., Kydd, R. A. & Brandt, K. K. Pillared clay minerals as catalysts and catalyst supports. Stud. Surf. Sci. Catal. 73, 287–290 (1992).
Amirbahman, A., Gfeller, M. & Furrer, G. Kinetics and mechanism of ligand-promoted decomposition of the Keggin Al13 polymer. Geochim. Cosmochim. Acta 64, 911–919 (2000).
Casey, W. H. Large aqueous aluminum-hydroxide molecules. Chem. Rev. 106, 1–16 (2006).
Rustad, J. R., Loring, J. S. & Casey, W. H. Oxygen-exchange pathways in aluminum polyoxocations. Geochim. Cosmochim. Acta 68, 3011–3017 (2004).
Villa, E. M. et al. Reaction dynamics of the decaniobate ([HxNb10O28](6−x)−) ion in water. Angew. Chem. Int. Ed. 47, 4844–4846 (2008).
Comba, P. & Helm, L. The solution structure and reactivity of decavanadate. Helv. Chim. Acta 71, 1406–1420 (1988).
Mega, T. L., Cortes, S. & Van Etten, R. L. The oxygen-18 isotope shift in carbon-13 nuclear magnetic resonance spectroscopy. 13. Oxygen exchange at anomeric carbon of D-glucose, D-mannose and D-fructose. J. Org. Chem. 55, 522–528 (1990).
Mega, T. L. & Van Etten, R. L. Oxygen exchange and bond cleavage reactions of carbohydrates studied using the oxygen-18 isotope shift in carbon-13 NMR spectroscopy. Basic Life Sci. 56, 85–93 (1990).
Bolhuis, P. G., Chandler, D., Dellago, C. & Geissler, P. L. Transition path sampling: throwing ropes over rough mountain passes, in the dark. Ann. Rev. Phys. Chem. 53, 291–318 (2002).
Antonio, M. R., Nyman, M. & Anderson, T. M. Direct observation of contact ion-pair formation in aqueous solution. Angew. Chem. Int. Ed. 48, 6136–6140 (2009).
Villa, E. M., Ohlin, C. A., Rustad, J. R. & Casey, W. H. Isotope-exchange dynamics in isostructural decametalates with profound differences in reactivity. J. Am. Chem. Soc. 131, 16488–16492 (2009).
Ohlin, C. A., Villa, E. M., Fettinger, J. C. & Casey, W. H. A new titanoniobate ion-completing the series [Nb10O28]6, [TiNb9O28]7− and [Ti2Nb8O28]8. Dalton T. 15, 2677–2678 (2009).
Casey, W. H. On the relative dissolution rates of some oxide and orthosilicate minerals. J. Colloid Interface Sci. 146, 586–589 (1991).
Rowsell, J. & Nazar, L. F. Speciation and thermal transformation in alumina sols: Structures of the polyhydroxyoxoaluminum cluster [Al30O8(OH)56(H2O)26]18+ and its δ-Keggin moiety. J. Am. Chem. Soc. 122, 3777–3778 (2000).
Müller, A., Diemann, E., Shah, S. Q. N., Kuhlmann, C. & Letzel Matthias, C. Soccer-playing metal oxide giant spheres: a first step towards patterning structurally well-defined nano-object collectives. Chem Commun. 440–441 (2002).
Balogh, E., Todea, A. M., Muller, A. & Casey, W. H. Rates of ligand exchange between >FeIII-OH2 functional groups on a nanometer-size aqueous cluster and bulk solution. Inorg. Chem. 46, 7087–7092 (2007).
Houston, J. R., Richens, D. T. & Casey, W. H. Distinct water-exchange mechanisms for similar trinuclear transition-metal clusters. Inorg. Chem. 45, 7962–7967 (2006).
Houston, J. R., Olmstead, M. O. & Casey, W. H. Substituent effects in five oxo-centered Rh(III) trimers. Inorg. Chem. 45, 7799–7805 (2006).
Acknowledgements
Support for this research came from the National Science Foundation via grant EAR 05015600 and from the US Department of Energy Office of Basic Energy Science via grant numbers DE-FG03-96ER 14629 and DE-FG03-02ER15693. Special thanks also goes to Phil Power who encouraged us to propose this article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ohlin, C., Villa, E., Rustad, J. et al. Dissolution of insulating oxide materials at the molecular scale. Nature Mater 9, 11–19 (2010). https://doi.org/10.1038/nmat2585
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2585
This article is cited by
-
Molecular insight into the initial hydration of tricalcium aluminate
Nature Communications (2024)
-
Geochemical modeling to aid experimental design for multiple isotope tracer studies of coupled dissolution and precipitation reaction kinetics
Acta Geochimica (2024)
-
Rapid oxygen exchange between hematite and water vapor
Nature Communications (2021)
-
Theoretical insights into the surface physics and chemistry of redox-active oxides
Nature Reviews Materials (2020)
-
Highly Conductive Graphene Electronics by Inkjet Printing
Journal of Electronic Materials (2020)