Abstract
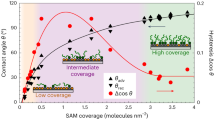
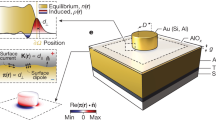
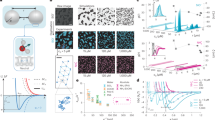
Natural surfaces are often structured with nanometre-scale domains, yet a framework providing a quantitative understanding of how nanostructure affects interfacial energy, γSL, is lacking. Conventional continuum thermodynamics treats γSL solely as a function of average composition, ignoring structure. Here we show that, when a surface has domains commensurate in size with solvent molecules, γSL is determined not only by its average composition but also by a structural component that causes γSL to deviate from the continuum prediction by a substantial amount, as much as 20% in our system. By contrasting surfaces coated with either molecular- (<2 nm) or larger-scale domains (>5 nm), we find that whereas the latter surfaces have the expected linear dependence of γSL on surface composition, the former show a markedly different non-monotonic trend. Molecular dynamics simulations show how the organization of the solvent molecules at the interface is controlled by the nanostructured surface, which in turn appreciably modifies γSL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bizzarri, A. R. & Cannistraro, S. Molecular dynamics of water at the protein-solvent interface. J. Phys. Chem. B 106, 6617–6633 (2002).
Hoffmann, M. R., Martin, S. T., Choi, W. Y. & Bahnemann, D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995).
Zhdanov, V. P. & Kasemo, B. Kinetics of electrochemical reactions: From single crystals to nm-sized supported particles. Surf. Sci. 521, L655–L661 (2002).
Stevens, M. M. & George, J. H. Exploring and engineering the cell surface interface. Science 310, 1135–1138 (2005).
Rogers, K. R. Principles of affinity-based biosensors. Mol. Biotech. 14, 109–129 (2000).
Willard, A. P. & Chandler, D. The role of solvent fluctuations in hydrophobic assembly. J. Phys. Chem. B 112, 6187–6192 (2008).
Oregan, B. & Gratzel, M. A low-cost, high-efficiency solar-cell based on dye-sensitized colloidal TiO2 films. Nature 353, 737–740 (1991).
Frauenfelder, H., Fenimore, P. W., Chen, G. & McMahon, B. H. Protein folding is slaved to solvent motions. Proc. Natl Acad. Sci. USA 103, 15469–15472 (2006).
Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 96, 1533–1554 (1996).
Love, J. C., Estroff, L. A., Kriebel, J. K., Nuzzo, R. G. & Whitesides, G. M. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 105, 1103–1169 (2005).
Mrksich, M. & Whitesides, G. M. Using self-assembled monolayers to understand the interactions of man-made surfaces with proteins and cells. Annu. Rev. Biophys. Biomol. Struct. 25, 55–78 (1996).
Stranick, S. J. et al. Nanometer-scale phase separation in mixed composition self-assembled monolayers. Nanotechnology 7, 438–442 (1996).
Pace, C. N., Treviño, S., Prabhakaran, E. & Scholtz, J. M. Protein structure, stability and solubility in water and other solvents. Phil. Trans. R. Soc. B 359, 1225–1235 (2004).
Stranick, S. J., Parikh, A. N., Tao, Y. T., Allara, D. L. & Weiss, P. S. Phase-separation of mixed-composition self-assembled monolayers into nanometer-scale molecular domains. J. Phys. Chem. 98, 7636–7646 (1994).
Centrone, A. et al. The role of nanostructure in the wetting behaviour of mixed-monolayer-protected metal nanoparticles. Proc. Natl Acad. Sci. USA 105, 9886–9891 (2008).
Jackson, A. M., Myerson, J. W. & Stellacci, F. Spontaneous assembly of subnanometre-ordered domains in the ligand shell of monolayer-protected nanoparticles. Nature Mater. 3, 330–336 (2004).
Singh, C. et al. Entropy-mediated patterning of surfactant-coated nanoparticles and surfaces. Phys. Rev. Lett. 99, 226106 (2007).
Carney, R. P. et al. Size limitations for the formation of ordered striped nanoparticles. J. Am. Chem. Soc. 130, 798–799 (2008).
Jackson, A. M., Hu, Y., Silva, P. J. & Stellacci, F. From homoligand- to mixed-ligand-monolayer-protected metal nanoparticles: A scanning tunnelling microscopy investigation. J. Am. Chem. Soc. 128, 11135–11149 (2006).
Dupré, A. Théorie Mécanique de La Chaleur (Gauthier-Villars, 1869).
Young, T. An essay on the cohesion of fluids. Phil. Trans. R. Soc. Lond. (1804).
Laibinis, P. E., Fox, M. A., Folkers, J. P. & Whitesides, G. M. Comparisons of self-assembled monolayers on silver and gold—mixed monolayers derived from Hs(Ch2)21x and Hs(Ch2)10y (X,Y=Ch3, Ch2oh) have similar properties. Langmuir 7, 3167–3173 (1991).
Wenzel, R. N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 28, 988–994 (1936).
Barthlott, W. & Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202, 1–8 (1997).
Chow, T. S. Wetting of rough surfaces. J. Phys. Condens. Matter 10, L445–L451 (1998).
Gennes, P. G. d. Soft Interfaces (Cambridge Univ. Press, 1994).
Cassie, A. B. D. & Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 40, 546–551 (1944).
Bohlen, H., Parry, A. O., Diaz-Herrera, E. & Schoen, M. Intrusion of fluids into nanogrooves. Eur. Phys. J. E 25, 103–115 (2008).
Schoen, M. Fluid bridges confined between chemically nanopatterned solid substrates. Phys. Chem. Chem. Phys. 10, 223–256 (2008).
Imabayashi, S., Gon, N., Sasaki, T., Hobara, D. & Kakiuchi, T. Effect of nanometre-scale phase separation on wetting of binary self-assembled thiol monolayers on Au(111). Langmuir 14, 2348–2351 (1998).
Maibaum, L. & Chandler, D. Segue between favorable and unfavorable solvation. J. Phys. Chem. B 111, 9025–9030 (2007).
Shipway, A. N., Katz, E. & Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. Chem. Phys. Chem. 1, 18–52 (2000).
Musick, M. D., Keating, C. D., Keefe, M. H. & Natan, M. J. Stepwise construction of conductive Au colloid multilayers from solution. Chem. Mater. 9, 1499–1501 (1997).
Brust, M., Bethell, D., Kiely, C. J. & Schiffrin, D. J. Self-assembled gold nanoparticle thin films with nonmetallic optical and electronic properties. Langmuir 14, 5425–5429 (1998).
Gao, L. C. & McCarthy, T. J. How Wenzel and Cassie were wrong. Langmuir 23, 3762–3765 (2007).
O’Shea, S. J., Welland, M. E. & Pethica, J. B. Atomic-force microscopy of local compliance at solid–liquid interfaces. Chem. Phys. Lett. 223, 336–340 (1994).
O’Shea, S. J. & Welland, M. E. Atomic force microscopy at solid–liquid interfaces. Langmuir 14, 4186–4197 (1998).
Lantz, M., Liu, Y. Z., Cui, X. D., Tokumoto, H. & Lindsay, S. M. Dynamic force microscopy in fluid. Surf. Interface Anal. 27, 354–360 (1999).
Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647 (2005).
Giovambattista, N., Debenedetti, P. G. & Rossky, P. J. Hydration behaviour under confinement by nanoscale surfaces with patterned hydrophobicity and hydrophilicity. J. Phys. Chem. C 111, 1323–1332 (2007).
Rajamani, S., Truskett, T. M. & Garde, S. Hydrophobic hydration from small to large lengthscales: Understanding and manipulating the crossover. Proc. Natl Acad. Sci. USA 102, 9475–9480 (2005).
Acknowledgements
F.S. is grateful to the Packard Foundation and to the National Science Foundation CAREER Award (DMR 06-45323) for their generous awards. J.J.K. was partly supported by a National Science Foundation Graduate Research Fellowship. K.V. acknowledges financial support from the Swiss National Science Foundation. C.S., H.J. and S.C.G. acknowledge financial support from the National Science Foundation under grant number CTS-0403633. S.M. acknowledges financial support from the University of London Central Research Fund, the Institute of Biomedical Engineering and the Natural Sciences and Engineering Research Council of Canada. M.M.S. thanks the European Research Council for financial support.
Author information
Authors and Affiliations
Contributions
J.J.K., K.V. and F.S. conceived the experiments. J.J.K. and S.M. fabricated the nanoparticle films and carried out contact angle measurements. J.J.K. fabricated the large-domain films and carried out contact angle measurements. K.V. carried out AFM measurements and analysed the AFM data, except roughness measurements (J.J.K.). J.J.K., K.V., S.M., M.M.S. and F.S. analysed the experimental data. C.S., H.J., P.K.G. and S.C.G. designed the simulations, H.J. carried out the simulations and C.S., H.J. and S.C.G. analysed the simulation data. J.J.K., K.V., C.S., H.J., S.C.G. and F.S. wrote most of the article. M.M.S. and S.M. contributed to manuscript revision.
Corresponding authors
Supplementary information
Supplementary Information
Supplementary Information (PDF 1926 kb)
Rights and permissions
About this article
Cite this article
Kuna, J., Voïtchovsky, K., Singh, C. et al. The effect of nanometre-scale structure on interfacial energy. Nature Mater 8, 837–842 (2009). https://doi.org/10.1038/nmat2534
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2534
This article is cited by
-
Multi-sulfonated ligands on gold nanoparticles as virucidal antiviral for Dengue virus
Scientific Reports (2020)
-
Cortical-like mini-columns of neuronal cells on zinc oxide nanowire surfaces
Scientific Reports (2019)
-
Quantitative 3D determination of self-assembled structures on nanoparticles using small angle neutron scattering
Nature Communications (2018)
-
Suppression of interdiffusion-induced voiding in oxidation of copper nanowires with twin-modified surface
Nature Communications (2018)
-
Mass spectrometry and Monte Carlo method mapping of nanoparticle ligand shell morphology
Nature Communications (2018)