Abstract
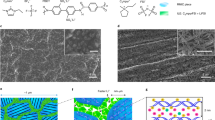
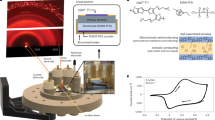
Polymer electrolytes have been studied extensively because uniquely they combine ionic conductivity with solid yet flexible mechanical properties, rendering them important for all-solid-state devices including batteries, electrochromic displays and smart windows1,2,3. For some 30 years, ionic conductivity in polymers was considered to occur only in the amorphous state above Tg. Crystalline polymers were believed to be insulators. This changed with the discovery of Li+ conductivity in crystalline poly(ethylene oxide)6:LiAsF6 (refs 4, 5). However, new crystalline polymer electrolytes have proved elusive, questioning whether the 6:1 complex has particular structural features making it a unique exception to the rule that only amorphous polymers conduct. Here, we demonstrate that ionic conductivity in crystalline polymers is not unique to the 6:1 complex by reporting several new crystalline polymer electrolytes containing different alkali metal salts (Na+, K+ and Rb+), including the best conductor poly(ethylene oxide)8:NaAsF6 discovered so far, with a conductivity 1.5 orders of magnitude higher than poly(ethylene oxide)6:LiAsF6. These are the first crystalline polymer electrolytes with a different composition and structures to that of the 6:1 Li+ complex.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Scrosati, B. (ed.) Applications of Electroactive Polymers (Chapman & Hall, 1993).
Gray, F. M. Polymer Electrolytes. RSC materials monographs (The Royal Society of Chemistry, 1997).
Tarascon, J.-M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001).
Gadjourova, Z., Andreev, Y. G., Tunstall, D. P. & Bruce, P. G. Ionic conductivity in crystalline polymer electrolytes. Nature 412, 520–523 (2001).
Christie, A. M., Lilley, S. J., Staunton, E., Andreev, Y. G. & Bruce, P. G. Increasing the conductivity of crystalline polymer electrolytes. Nature 433, 50–53 (2005).
Fenton, D. E., Parker, J. M. & Wright, P. V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14, 589 (1973).
Armand, M. B., Chabango, J. M. & Duclot, M. J. in Fast Ion Transport in Solids (eds Vashishta, P., Mundy, J. N. & Shenoy, G. K.) 131–136 (North-Holland, 1979).
Berthier, C., Gorecki, W., Minier, M., Armand, M. B., Chabagno, J. M. & Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts—poly(ethylene oxide) adducts. Solid State Ion. 11, 91–95 (1983).
Ratner, M. A. in Polymer Electrolytes Reviews—1 (eds MacCallum, J. R. & Vincent, C. A.) 173–236 (Elsevier Applied Science, 1987).
Ratner, M. A. & Shriver, D. F. Ion-transport in solvent-free polymers. Chem. Rev. 88, 109–124 (1988).
Armand, M. B. Polymers with ionic conductivity. Adv. Mater. 2, 278–286 (1990).
Hutchison, J. C., Bissessur, R. & Shriver, D. F. New polyphosphazene-clay and cryptand-clay intercalates. Nanotechnology 622, 262–272 (1996).
Tokuda, H. & Watanabe, M. Characterization and ionic transport properties of nano-composite electrolytes containing a lithium salt of a superweak aluminate anion. Electrochim. Acta 48, 2085–2091 (2003).
Golodnitsky, D. et al. New generation of ordered polymer electrolytes for lithium batteries. Electrochem. Solid-State Lett. 7, A412–A415 (2004).
Wright, P. V., Zheng, Y., Bhatt, D., Richardson, T. & Ungar, G. Supramolecular order in new polymer electrolytes. Polym. Int. 47, 34–42 (1998).
Evans, J., Vincent, C. A. & Bruce, P. G. Electrochemical measurement of transference numbers in polymer electrolytes. Polymer 28, 2324–2328 (1987).
Bruce., P. G. (ed.) Solid State Electrochemistry (Cambridge Univ. Press, 1995).
Gjikaj, M. & Adam, A. Complexation of alkali triflates by crown ethers: Synthesis and crystal structure of [Na(12-crown-4)2][SO3CF3], [Na(5-crown-5)2][SO3CF3], [Rb(18-crown-6)2][SO3CF3], and [Cz(18-crown-6)2][SO3CF3]. Z. Anorg. Allg. Chem. 632, 2475–2480 (2006).
Bush, M. A. & Truter, M. R. Crystal structures of complexes between alkali-metal salts and cyclic polyethers, part II. Complex formed from sodium bromide and 2,3,11,12-dibenzo-1,4,7,10,13,16-hexaoxacyclo-octadeca-2,11-diene (‘Di-benzo-18-crown-6’). J. Chem. Soc. B 1440–1446 (1971).
Mason, E. & Eick, H. A. Structure of a 1:2 complex of sodium perchlorate and 1,4,7,10-tetraoxacyclododecane (12-crown-4). Acta Cryst. B 38, 1821–1823 (1982).
Brandell, D., Liivat, A., Aabloo, A. & Thomas, J. O. Molecular dynamics simulation of the crystalline short-chain polymer system LiPF6:PEO6 (Mw∼1,000). J. Mater. Chem. 15, 4338–4345 (2005).
Liivat, A., Brandell, D. & Thomas, J. O. A molecular dynamics study of ion-conduction mechanisms in crystalline low−MwLiPF6:PEO6 . J. Mater. Chem. 17, 3938–3946 (2007).
Sauvage, F., Laffont, L., Tarascon, J.-M. & Baudrin, E. Study of the insertion/deinsertion mechanism of sodium into Na0.44MnO2 . Inorg. Chem. 46, 3289–3294 (2007).
Doeff, M. M., Peng, M. Y., Ma, Y. & De Jonghe, L. C. Orthorhombic NaxMnO2 as a cathode material for secondary sodium and lithium polymer batteries. J. Electrochem. Soc. 141, L145–L147 (1994).
Staunton, E., Andreev, Y. G. & Bruce, P. G. Factors influencing the conductivity of crystalline polymer electrolytes. Faraday Discuss. 134, 143–156 (2007).
Zhang, C., Staunton, E., Andreev, Y. G. & Bruce, P. G. Raising the conductivity of crystalline polymer electrolytes by aliovalent doping. J. Am. Chem. Soc. 127, 18305–18308 (2005).
Gray, F. M., McCallum, J. R. & Vincent, C. A. USA Patent Appl. 8 610 049, May 1986.
Sheldrick, G. M. SHELXTL 6.14 (Bruker AXS, Madison, 2004).
Acknowledgements
The authors are grateful to J. K. Cockcroft, University College London, for collecting variable-temperature PXRD data. P.G.B. is indebted to the EPSRC and the EU for financial support.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 362 kb)
Rights and permissions
About this article
Cite this article
Zhang, C., Gamble, S., Ainsworth, D. et al. Alkali metal crystalline polymer electrolytes. Nature Mater 8, 580–584 (2009). https://doi.org/10.1038/nmat2474
Issue Date:
DOI: https://doi.org/10.1038/nmat2474
This article is cited by
-
Structural and conductivity investigations of composite polymer electrolytes based on poly(oxyethylene)
Applied Physics A (2023)
-
Lithium polymer electrolytes for novel batteries application: the review perspective
Applied Physics A (2023)
-
A highly ionic conductive succinonitrile-based composite solid electrolyte for lithium metal batteries
Nano Research (2022)
-
Recent advances in NASICON-type oxide electrolytes for solid-state sodium-ion rechargeable batteries
Ionics (2022)
-
High sodium ionic conductivity in PEO/PVP solid polymer electrolytes with InAs nanowire fillers
Scientific Reports (2021)