Abstract
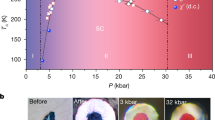
It is widely accepted that ice, no matter what phase, is unable to incorporate large amounts of salt into its structure. This conclusion is based on the observation that on freezing of salt water, ice expels the salt almost entirely as brine. Here, we show that this behaviour is not an intrinsic physico-chemical property of ice phases. We demonstrate by neutron diffraction that substantial amounts of dissolved LiCl can be built homogeneously into the ice VII structure if it is produced by recrystallization of its glassy (amorphous) state under pressure. Such ‘alloyed’ ice VII has significantly different structural properties compared with pure ice VII, such as an 8% larger unit cell volume, 5 times larger displacement factors, an absence of a transition to an ordered ice VIII structure and plasticity. Our study suggests that there could be a whole new class of ‘salty’ high-pressure ice forms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pentrenko, V. F. & Whitworth, R. W. Physics of Ice (Oxford Univ. Press, 1999).
Jal, J. F., Soper, A. K., Carmona, P. & Dupuy, J. Microscopic structure of LiCl·6D2O in glassy and liquid phases. J. Phys. Condens. Matter 3, 551–567 (1991).
Prevel, B., Dupuy-Philon, J., Jal, J. F., Legrand, J. F. & Chieux, P. Structural relation in supercooled glass-forming solutions: A neutron spin-echo study of LiCl·6D2O. J. Phys. Condens. Matter 6, 1279–1290 (1994).
Nelmes, R. J. et al. Annealed high density amorphous ice under pressure. Nature Phys. 2, 414–418 (2006).
Hönnerschneid, A., Nuss, J., Mühle, C. & Jansen, M. Die Kristallstrukturen der Monohydrate von Lithiumchlorid und Lithiumbromid. Z. Anorg. Allg. Chem. 629, 312–316 (2003).
Culot, J. P., Piret, P. & Van Meerssche, M. Structure de NaBr·H2O. Bull. Soc. Fr. Minér. Cristallogr. 85, 282–289 (1962).
Klewe, B. & Pedersen, B. The crystal structure of sodium chloride dihydrate. Acta Crystallogr. B 30, 2363–2371 (1974).
Verbist, J., Piret, P. & Van Meerssche, M. Structure cristalline et protonique de l’iodure de sodium dihydraté NaI·2H2O. Bull. Soc. Fr. Minér. Cristallogr. 93, 509–514 (1970).
Jorgensen, J. D. & Worlton, T. G. Disordered structure of D2O ice VII from in situ neutron powder diffraction. J. Chem. Phys. 83, 329–333 (1985).
Metzger, T. H. & Trautmann, C. Strong atomic displacements around nitrogen in tantalum determined by energy-dispersive X-ray diffraction. Z. Phys. B 62, 63–70 (1985).
Schubert, U., Metzger, H. & Peisl, J. Diffuse X-ray scattering from interstitial nitrogen in niobium: I. Huang diffuse scattering. J. Phys. F 14, 2457–2466 (1984).
Besson, J. M. et al. Variation of interatomic distances in ice VIII to 10 GPa. Phys. Rev. B 49, 12540–12550 (1994).
Takii, Y., Koga, K. & Tanaka, H. A plastic phase of water from computer simulations. J. Chem. Phys. 128, 204501–204508 (2008).
Vos, W. L., Finger, L. W., Hemley, R. J. & Mao, H.-K. Novel H2–H2O clathrates at high pressures. Phys. Rev. Lett. 71, 3150–3153 (1993).
Londono, D., Kuhs, W. F. & Finney, J. L. Enclathration of helium in ice II: The first helium-hydrate. Nature 332, 141–142 (1988).
Hartwig, P. & Weppner, W. Ionic conductivities of lithium-halide-based quaternary compounds. Solid State Ion. 3/4, 249–254 (1981).
Poulsen, F. W. Ionic conductivity of solid lithium iodine and its monohydrates. Solid State Ion. 2, 53–57 (1981).
Frank, M. R. et al. Experimental study of the NaCl–H2O system up to 28 GPa: Implications of ice-rich planetary bodies. Phys. Earth Planet. Interiors 155, 152–162 (2006).
Zimmer, Ch., Khurana, K. K. & Kivelson, M. G. Subsurface oceans on Europa and Callisto: Constraints from Galileo magnetometer observations. Icarus 147, 329–347 (2000).
McCord, T. B., Hansen, G. B. & Hibbitts, C. A.. Hydrated salt minerals on Ganymede’s surface: Evidence of an ocean below. Science 292, 1523–1525 (2001).
Lorenz, R. D. et al. Titan’s rotation reveals an internal ocean and changing zonal winds. Science 319, 1649–1651 (2008).
Hansen, T. C., Henry, P. F., Fischer, H. E., Torregrossa, J. & Convert, P. The D20 instrument at the ILL: A versatile high-intensity two-axis neutron diffractometer. Meas. Sci. Tech. 19, 034001 (2008).
Strässle, Th., Klotz, S., Hamel, G., Koza, M. & Schober, H. Experimental evidence for a crossover between two distinct mechanisms of amorphisation in ice under pressure. Phys. Rev. Lett. 99, 175501–175504 (2007).
Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 192, 55–69 (1993).
Besson, J. M. et al. Structural instability in ice VIII under pressure. Phys. Rev. Lett. 78, 3141–3144 (1997).
Chialvo, A. A. & Simonson, J. M. Ion association in aqueous LiCl solutions at high concentration: Predicted results via molecular simulation. J. Chem. Phys. 124, 154509 (2006).
Acknowledgements
The authors are very grateful to W.F. Kuhs for pointing out the issue of plasticity in ice VII. We thank J.-L. Laborier (ILL), G. Hamel and J. Philippe for technical assistance and advice, as well as R. Pick, F. Frey and M. Schmidbauer for helpful discussions. A.M.S. acknowledges support from the French National Supercomputing Facility IDRIS, where all of the calculations have been carried out under the projects CP9-71387 and CP9-81387. This work is partially based on experiments carried out at the Swiss spallation source SINQ, Paul Scherrer Institute, Villigen, Switzerland.
Author information
Authors and Affiliations
Contributions
S.K., L.E.B, T.S. and T.C.H. carried out the experiments and A.M.S. the calculations. S.K. wrote the paper.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 353 kb)
Rights and permissions
About this article
Cite this article
Klotz, S., Bove, L., Strässle, T. et al. The preparation and structure of salty ice VII under pressure. Nature Mater 8, 405–409 (2009). https://doi.org/10.1038/nmat2422
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2422
This article is cited by
-
Salty ice and the dilemma of ocean exoplanet habitability
Nature Communications (2022)
-
Stability of high-temperature salty ice suggests electrolyte permeability in water-rich exoplanet icy mantles
Nature Communications (2022)
-
Large Ocean Worlds with High-Pressure Ices
Space Science Reviews (2020)
-
Ice VII from aqueous salt solutions: From a glass to a crystal with broken H-bonds
Scientific Reports (2016)