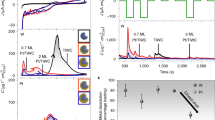
Abstract
Recent advances in colloidal synthesis enabled the precise control of the size, shape and composition of catalytic metal nanoparticles, enabling their use as model catalysts for systematic investigations of the atomic-scale properties affecting catalytic activity and selectivity. The organic capping agents stabilizing colloidal nanoparticles, however, often limit their application in high-temperature catalytic reactions. Here, we report the design of a high-temperature-stable model catalytic system that consists of a Pt metal core coated with a mesoporous silica shell (Pt@mSiO2). Inorganic silica shells encaged the Pt cores up to 750 ∘C in air and the mesopores providing direct access to the Pt core made the Pt@mSiO2 nanoparticles as catalytically active as bare Pt metal for ethylene hydrogenation and CO oxidation. The high thermal stability of Pt@mSiO2 nanoparticles enabled high-temperature CO oxidation studies, including ignition behaviour, which was not possible for bare Pt nanoparticles because of their deformation or aggregation. The results suggest that the Pt@mSiO2 nanoparticles are excellent nanocatalytic systems for high-temperature catalytic reactions or surface chemical processes, and the design concept used in the Pt@mSiO2 core–shell catalyst can be extended to other metal/metal oxide compositions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Science 299, 1688–1691 (2003).
Schlögl, R. & Abd Hamid, S. B. Nanocatalysis: mature science revisited or something really new? Angew. Chem. Int. Ed. 43, 1628–1637 (2004).
Somorjai, G. A., Contreras, A. M., Montano, M. & Rioux, R. M. Clusters, surfaces, and catalysis. Proc. Natl Acad. Sci. USA 103, 10577–10583 (2006).
Thomas, J. M. Heterogeneous catalysis: Enigmas, illusions, challenges, realities, and emergent strategies of design. J. Chem. Phys. 128, 182502 (2008).
Somorjai, G. A. Introduction to Surface Chemistry and Catalysis (Wiley, 1994).
Over, H. et al. Atomic-scale structure and catalytic reactivity of the RuO2(110) surface. Science 287, 1474–1476 (2000).
Greeley, J. & Mavrikakis, M. Alloy catalysts designed from first principles. Nature Mater. 3, 810–815 (2004).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Roucoux, A., Schulz, J. & Patin, H. Reduced transition metal colloids: A novel family of reusable catalysts? Chem. Rev. 102, 3757–3778 (2002).
Park, J., Joo, J., Kwon, S. G., Jang, Y. & Hyeon, T. Synthesis of monodisperse spherical nanocrystals. Angew. Chem. Int. Ed. 46, 4630–4660 (2007).
Tao, A. R., Habas, S. & Yang, P. Shape control of colloidal metal nanocrystals. Small 4, 310–325 (2008).
Ahmadi, T. S., Wang, Z. L., Green, T. C., Henglein, A. & El-Sayed, M. A. Shape-controlled synthesis of colloidal platinum nanoparticles. Science 272, 1924–1926 (1996).
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Song, H. et al. Hydrothermal growth of mesoporous SBA-15 silica in the presence of PVP-stabilized Pt nanoparticles: Synthesis, characterization, and catalytic properties. J. Am. Chem. Soc. 128, 3027–3037 (2006).
Narayanan, R. & El-Sayed, M. A. Shape-dependent catalytic activity of platinum nanoparticles in colloidal solution. Nano Lett. 4, 1343–1348 (2004).
Tian, N., Zou, Z.-Y., Sun, S.-G., Ding, Y. & Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316, 732–735 (2007).
Bratlie, K. M., Lee, H., Komvopoulos, K., Yang, P. & Somorjai, G. A. Platinum nanoparticle shape effects on benzene hydrogenation selectivity. Nano Lett. 7, 3097–3101 (2007).
Alayoglu, S., Nilekar, A. U., Mavrikakis, M. & Eichhorn, B. Ru–Pt core–shell nanoparticles for preferential oxidation of carbon monoxide in hydrogen. Nature Mater. 7, 333–338 (2008).
Park, J. Y., Zhang, Y., Grass, M., Zhang, T. & Somorjai, G. A. Tuning of catalytic CO oxidation by changing composition of Rh–Pt bimetallic nanoparticles. Nano Lett. 8, 673–677 (2008).
Langmuir, I. The mechanism of the catalytic action of platinum in the reactions 2CO+O2=2CO2 and 2H2+O2=2H2O. Trans. Faraday Soc. 17, 621–654 (1922).
Campbell, C. T., Ertl, G., Kuipers, H. & Segner, J. A molecular beam study of the catalytic oxidation of CO on a Pt(111) surface. J. Chem. Phys. 73, 5862–5873 (1980).
Berlowitz, P. J., Peden, C. H. F. & Goodman, D.W. Kinetics of CO oxidation on single-crystal Pd, Pt, and Ir. J. Phys. Chem. 92, 5213–5221 (1988).
Chen, M. S. et al. Highly active surfaces for CO oxidation on Rh, Pd, and Pt. Surf. Sci. 601, 5326–5331 (2007).
Su, X., Cremer, P. S., Shen, Y. R. & Somorjai, G. A. High-pressure CO oxidation on Pt(111) monitored with infrared-visible sum frequency generation (SFG). J. Am. Chem. Soc. 119, 3994–4000 (1997).
McCrea, K. R., Parker, J. S. & Somorjai, G. A. The role of carbon deposition from CO dissociation on platinum crystal surfaces during catalytic CO oxidation: Effects on turnover rate, ignition temperature, and vibrational spectra. J. Phys. Chem. 106, 10854–10863 (2002).
Zaera, F. The surface chemistry of hydrocarbon partial oxidation catalysis. Catal. Today 81, 149–157 (2003).
Huber, G. W. & Corma, A. Synergies between bio- and oil refineries for the production of fuels from biomass. Angew. Chem. Int. Ed. 46, 7184–7201 (2007).
Ciuparu, D., Lyubovsky, M. R., Altman, E., Pfefferle, L. D. & Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 44, 593–649 (2002).
Somorjai, G. A. & Rioux, R. M. High technology catalysts towards 100% selectivity. Fabrication, characterization and reaction studies. Catal. Today 100, 201–215 (2005).
Lee, H. et al. Morphological control of catalytically active platinum nanocrystals. Angew. Chem. Int. Ed. 45, 7824–7828 (2006).
Kresge, C. T., Leonowicz, M. E., Roth, W. J., Vartuli, J. C. & Beck, J. S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal mechanism. Nature 359, 710–712 (1992).
Corma, A. From microporous to mesoporous molecular sieve materials and their use in catalysis. Chem. Rev. 97, 2373–2419 (1997).
Kim, M., Sohn, K., Na, H. B. & Hyeon, T. Synthesis of nanorattles composed of gold nanoparticles encapsulated in mesoporous carbon and polymer shells. Nano Lett. 2, 1383–1387 (2002).
Nooney, R. I., Thirunavukkarasu, D., Chen, Y., Josephs, R. & Ostafin, A. E. Self-assembly of mesoporous nanoscale silica/gold composites. Langmuir 19, 7628–7637 (2003).
Botella, P., Corma, A. & Navarro, M. T. Single gold nanoparticles encapsulated in monodispersed regular spheres of mesostructured silica produced by pseudomorphic transformation. Chem. Mater. 19, 1979–1983 (2007).
van Hardeveld, R. & Hartog, F. The statistics of surface atoms and surface sites on metal crystals. Surf. Sci. 15, 189–230 (1969).
Yu, R., Song, H., Zhang, X.-F. & Yang, P. Thermal wetting of platinum nanocrystals on silica surface. J. Phys. Chem. B 109, 6940–6943 (2005).
Yin, Y. et al. Formation of hollow nanocrystals through nanoscale Kirkendall effect. Science 304, 711–714 (2004).
Lin, K.-J., Chen, L.-J., Prasad, M. R. & Cheng, C.-Y. Core–shell synthesis of a novel, spherical, mesoporous silica/platinum nanocomposite: Pt/PVP@MCM-41. Adv. Mater. 16, 1845–1849 (2004).
Ikeda, S. et al. Ligand-free platinum nanoparticles encapsulated in a hollow porous carbon shell as a highly active heterogeneous hydrogenation catalyst. Angew. Chem. Int. Ed. 45, 7063–7066 (2006).
Arnal, P. M., Comotti, M. & Schüth, F. High-temperature-stable catalysts by hollow sphere encapsulation. Angew. Chem. Int. Ed. 45, 8224–8227 (2006).
Lee, J., Park, J. C. & Song, H. A nanoreactor framework of a Au@SiO2 yolk/shell structure for catalytic reduction of p-nitrophenol. Adv. Mater. 20, 1523–1528 (2008).
Yu, K., Wu, Z., Zhao, Q., Li, B. & Xie, Y. High-temperature-stable Au@SnO2 core/shell supported catalyst for CO oxidation. J. Phys. Chem. C 112, 2244–2247 (2008).
Liz-Marzán, L. M., Giersig, M. & Mulvaney, P. Synthesis of nanosized gold-silica core–shell particles. Langmuir 12, 4329–4335 (1996).
Lu, Y., Yin, Y., Li, Z.-Y. & Xia, Y. Synthesis and self-assembly of Au@SiO2 core–shell colloids. Nano Lett. 2, 785–788 (2002).
Gorelikov, I. & Matsuura, N. Single-step coating of mesoporous silica on cetyltrimethyl ammonium bromide-capped nanoparticles. Nano Lett. 8, 369–373 (2008).
Schmidt-Winkel, P. et al. Microemulsion templating of siliceous mesostructured cellular foams with well-defined ultralarge mesopores. Chem. Mater. 12, 686–696 (2000).
Rioux, R. M., Song, H., Hoefelmeyer, J. D., Yang, P. & Somorjai, G. A. High-surface-area catalyst design: Synthesis, characterization, and reaction studies of platinum nanoparticles in mesoporous SBA-15 silica. J. Phys. Chem. B 109, 2192–2202 (2005).
Acknowledgements
This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geological and Biosciences and Division of Materials Sciences and Engineering of the U.S. Department of Energy under contract No. DE-AC02-05CH11231. We thank A. P. Alivisatos and his group for the use of TEM and XRD equipment. We also thank J. N. Kuhn, Y.-w. Jun and J. Park for helpful comments and S. M. Ko for illustrations in Fig. 1.
Author information
Authors and Affiliations
Contributions
G.A.S., P.Y., S.H.J. and J.Y.P. designed the research. S.H.J, C.-K.T. and Y.Y. carried out the synthesis and characterizations of nanoparticles. J.Y.P. and S.H.J. carried out catalysis experiments. S.H.J., J.Y.P., P.Y. and G.A.S. wrote the paper.
Corresponding author
Supplementary information
Supplementary Information
Supplementary Information (PDF 1802 kb)
Rights and permissions
About this article
Cite this article
Joo, S., Park, J., Tsung, CK. et al. Thermally stable Pt/mesoporous silica core–shell nanocatalysts for high-temperature reactions. Nature Mater 8, 126–131 (2009). https://doi.org/10.1038/nmat2329
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat2329
This article is cited by
-
Synthesis of core@shell catalysts guided by Tammann temperature
Nature Communications (2024)
-
Core–shell CoN@Co ultra-stable nanoparticles on biochar for contamination remediation in water and soil
Carbon Research (2024)
-
Emulsion-oriented assembly for Janus double-spherical mesoporous nanoparticles as biological logic gates
Nature Chemistry (2023)
-
Strategies for Designing the Catalytic Environment Beyond the Active site of Heterogeneous Supported Metal Catalysts
Topics in Catalysis (2023)
-
Ultimate structures in catalysis: Single atoms, subnano-clusters, and electrons
Science China Materials (2023)