Abstract
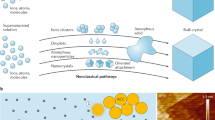
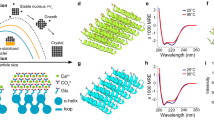
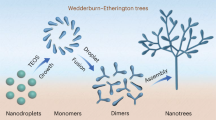
Diatoms, shells, bones and teeth are exquisite examples of well-defined structures, arranged from nanometre to macroscopic length scale, produced by natural biomineralization using organic templates to control the growth of the inorganic phase1,2,3,4,5,6. Although strategies mimicking Nature have partially succeeded in synthesizing human-designed bio-inorganic composite materials7,8,9,10, our limited understanding of fundamental mechanisms has so far kept the level of hierarchical complexity found in biological organisms out of the chemists’ reach11. In this letter, we report on the synthesis of unprecedented double-walled silica nanotubes with monodisperse diameters that self-organize into highly ordered centimetre-sized fibres. A unique synergistic growth mechanism is elucidated by the combination of light and electron microscopy, synchrotron X-ray diffuse scattering and Raman spectroscopy. Following this growth mechanism, macroscopic bundles of nanotubules result from the kinetic cross-coupling of two molecular processes: a dynamical supramolecular self-assembly and a stabilizing silica mineralization. The feedback actions between the template growth and the inorganic deposition are driven by a mutual electrostatic neutralization. This ‘dynamical template’ concept can be further generalized as a rational preparation scheme for materials with well-defined multiscale architectures and also as a fundamental mechanism for growth processes in biological systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lowenstam, H. A. & Weiner, S. On Biomineralisation (Oxford Univ. Press, New York, 1989).
Sundar, V. C., Yablon, A. D., Grazul, J. L., Ilan, M. & Aizenberg, J. Fibre-optical features of a glass sponge. Nature 424, 899–900 (2003).
Politi, Y., Arad, T., Klein, E., Weiner, S. & Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 306, 1161–1164 (2004).
Aizenberg, J. & Hendlen, G. Designing efficient microlens arrays: Lessons from Nature. J. Mater. Chem. 14, 2066–2072 (2004).
Aizenberg, J. et al. Skeleton of Euplectella sp.: Structural hierarchy from the nanoscale to the macroscale. Science 309, 275–278 (2005).
Aizenberg, J., Sundar, V. C., Yablon, A. D., Weaver, J. C. & Chen, G. Biological glass fibers: Correlation between optical and structural properties. Proc. Natl Acad. Sci. USA 101, 3358–3363 (2004).
Mann, S. Biomineralization. Principles and Concepts in Bioinorganic Materials Chemistry (Oxford Univ. Press, Oxford, 2001).
Dujardin, E. & Mann, S. Bio-inspired materials chemistry. Adv. Mater. 14, 775–788 (2002).
Yang, H., Coombs, N. & Ozin, G. A. Morphogenesis of shapes and surface patterns in mesoporous silica. Nature 386, 692–695 (1997).
Hartgerink, J. D., Beniash, E. & Stupp, S. I. Self-assembly and mineralization of peptide–amphiphile nanofibers. Science 294, 1684–1688 (2001).
Sanchez, C., Arribat, H. & Giraud-Guille, M. M. Biomimetism and bioinspiration as tools for the design of innovative materials systems. Nature Mater. 4, 277–288 (2005).
Woesz, A. et al. Micromechanical properties of biological silica skeletons of deep-sea sponges. J. Mater. Res. 21, 2068–2078 (2006).
Cha, J. N. et al. Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro. Proc. Natl Acad. Sci. USA 96, 361–365 (1999).
Kröger, N., Lorenz, S., Brunner, E. & Sumper, M. Self-assembly of highly phosphorylated silaffins and their function in biosilica morphogenesis. Science 298, 584–586 (2002).
Hukkamaki, J. & Pakkanen, T. T. Amorphous silica materials prepared by neutral templating route using amine-terminated templates. Micro. Meso. Mater. 65, 189–196 (2003).
Van Bommel, K. J. C. & Shinkai, S. Silica transcription in the absence of a solution catalyst: The surface mechanism. Langmuir 18, 4544–4548 (2002).
Shimizu, T., Masuda, M. & Minamikawa, H. Supramolecular nanotube architectures based on amphiphilic molecules. Chem. Rev. 105, 1401–1443 (2005).
Schroder, H. C. et al. Co-expression and functional interaction of silicatein with galectin—Matrix-guided formation of siliceous spicules in the marine demosponge Suberites domuncula. J. Biol. Chem. 281, 12001–12009 (2006).
Schroder, H. C. et al. Apposition of silica lamellae during growth of spicules in the demosponge Suberites domuncula: Biological/biochemical studies and chemical/biomimetical confirmation. J. Struct. Biol. (in the press).
Valéry, C. et al. Biomimetic organization: Octapeptide self-assembly into nanotubes of viral capsid-like dimension. Proc. Natl Acad. Sci. USA 100, 10258 (2003).
Valéry, C. et al. Self-association process of a peptide in solution: From beta-sheet filaments to large embedded nanotubes. Biophys. J. 86, 2484 (2004).
Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives (VCH, Weinheim, 1995).
Lesaint, C., Lebeau, B., Marichal, C., Patarin, J. & Zana, R. Fluorescence probing investigation of the mechanism of formation of MSU-type mesoporous silica prepared in fluoride medium. Langmuir 21, 8923–8929 (2005).
Israelachvili, J. Intramolecular and Surface Forces 2nd edn (Academic, San Diego, 1992).
Brinker, C. J. & Scherer, G. W. Sol–Gel Science. The Physics and Chemistry of Sol–Gel Processing (Academic, New York, 1990).
Ji, Q. et al. Direct sol–gel replication without catalyst in an aqueous gel system: From a lipid nanotube with a single bilayer wall to a uniform silica hollow cylinder with an ultrathin wall. Chem. Mater. 16, 250–254 (2004).
Ji, Q., Iwaura, R. & Shimizu, T. Controlling wall thickness of silica nanotubes within 4-nm precision. Chem. Lett. 33, 504–505 (2004).
Adachi, M., Harada, T. & Harada, M. Formation of huge length silica nanotubes by a templating mechanism in the laurylamine/tetraethoxysilane system. Langmuir 15, 7097–7100 (1999).
Uriz, M. J., Turon, X., Becerro, M. A. & Agell, G. Siliceous spicules and skeleton frameworks in sponges: Origin, diversity, ultrastructural patterns and biological functions. Microsc. Res. Technol. 62, 279–299 (2003).
Croce, G. et al. Structural characterization of siliceous spicules from marine sponges. Biophys. J. 86, 526–534 (2004).
Acknowledgements
Beaufour-IPSEN is acknowledged for providing the peptide and European Synchrotron Radiation Facility (ESRF) for allocating beam time (SC801). We are thankful to T. Narayanan for her support during preliminary experiments on the ID02 beamline at the ESRF synchrotron. E. Henry is acknowledged for the capillary picture. This work was supported by the Centre National de la Recherche Scientifique (AC Nanosciences–Nanotechnologies), by a research ministry fellowship (E.P.).
Author information
Authors and Affiliations
Contributions
F.A. designed the research, with additional contributions from E.D. and M.P. C.V., V.M.-A., M.P. and F.A. initiated the project. E.P., E.D. and A.C. performed TEM experiments. E.P., T.W., A.R. and F.A. performed SAXS experiments. E.P. and A.M. performed Raman experiments. E.P., E.D., M.P. and F.A. analysed data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary information and figures S1-S5 (PDF 139 kb)
Rights and permissions
About this article
Cite this article
Pouget, E., Dujardin, E., Cavalier, A. et al. Hierarchical architectures by synergy between dynamical template self-assembly and biomineralization. Nature Mater 6, 434–439 (2007). https://doi.org/10.1038/nmat1912
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1912
This article is cited by
-
Crystal engineering of quercetin via combined adsorption of polyvinylpyrrolidone and tannin
Korean Journal of Chemical Engineering (2023)
-
Damage-tolerant material design motif derived from asymmetrical rotation
Nature Communications (2022)
-
Effect of Silica Nanotube Surface Modification on the Physical Properties of Nanocomposites with Poly(methyl methacrylate)
Macromolecular Research (2018)
-
Chemically and thermally stable silica nanowires with a β-sheet peptide core for bionanotechnology
Journal of Nanobiotechnology (2016)
-
Lanreotide Depot: An Antineoplastic Treatment of Carcinoid or Neuroendocrine Tumors
Journal of Gastrointestinal Cancer (2016)