Abstract
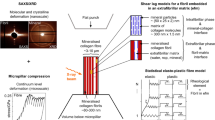
Nanomechanical heterogeneity is expected to influence elasticity, damage, fracture and remodelling of bone. Here, the spatial distribution of nanomechanical properties of bone is quantified at the length scale of individual collagen fibrils. Our results show elaborate patterns of stiffness ranging from ∼2 to 30 GPa, which do not correlate directly with topographical features and hence are attributed to underlying local structural and compositional variations. We propose a new energy-dissipation mechanism arising from nanomechanical heterogeneity, which offers a means for ductility enhancement, damage evolution and toughening. This hypothesis is supported by computational simulations that incorporate the nanoscale experimental results. These simulations predict that non-uniform inelastic deformation over larger areas and increased energy dissipation arising from nanoscale heterogeneity lead to markedly different biomechanical properties compared with a uniform material. The fundamental concepts discovered here are applicable to a broad class of biological materials and may serve as a design consideration for biologically inspired materials technologies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weiner, S. & Wagner, H. D. The material bone: Structure–mechanical function relations. Annu. Rev. Mater. Sci. 28, 271–298 (1998).
Currey, J. Structural heterogeneity in bone: Good or bad? J. Musculoskelet. Neuronal Interact. 5, 317 (2005).
Lakes, R. S. Materials with structural hierarchy. Nature 361, 511–515 (1993).
Morgan, E. F., Bayraktar, H. H. & Keaveny, T. M. Trabecular bone modulus–density relationships depend on anatomic site. J. Biomech. 36, 897–904 (2003).
Pope, M. H. & Outwater, J. O. Mechanical properties of bone as a function of position and orientation. J. Biomech. 7, 61–66 (1974).
Skedros, J. G., Sorenson, S. M., Takano, Y. & Turner, C. H. Dissociation of mineral and collagen orientations may differentially adapt compact bone for regional loading environments: Results from acoustic velocity measurements in deer calcanei. Bone 39, 143–151 (2006).
Rho, J. Y., Roy, M. E., Tsui, T. Y. & Pharr, G. M. Elastic properties of microstructural components of human bone tissue as measured by nanoindentation. J. Biomed. Mater. Res. 45, 48–54 (1999).
Gupta, H. S. et al. Mechanical modulation at the lamellar level in osteonal bone. J. Mater. Res. 21, 1913–1921 (2006).
Martin, R. B. & Burr, D. B. Structure, Function and Adaptation of Compact Bone (Raven, New York, 1989).
Rho, J. Y., Zioupos, P., Currey, J. D. & Pharr, G. M. Microstructural elasticity and regional heterogeneity in human femoral bone of various ages examined by nano-indentation. J. Biomech. 35, 189–198 (2002).
Balooch, G. et al. TGF-beta regulates the mechanical properties and composition of bone matrix. Proc. Natl Acad. Sci. 102, 18813–18818 (2005).
Zysset, P. K., Guo, X. E., Hoffler, C. E., Moore, K. E. & Goldstein, S. A. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 32, 1005–1012 (1999).
Catanese, J. 3rd, Iverson, E. P., Ng, R. K. & Keaveny, T. M. Heterogeneity of the mechanical properties of demineralized bone. J. Biomech. 32, 1365–1369 (1999).
Peterlik, H., Roschger, P., Klaushofer, K. & Fratzl, P. From brittle to ductile fracture of bone. Nature Mater. 5, 52–55 (2006).
Phelps, J. B., Hubbard, G. B., Wang, X. & Agrawal, C. M. Microstructural heterogeneity and the fracture toughness of bone. J. Biomed. Mater. Res. 51, 735–741 (2000).
Jaasma, M. J., Bayraktar, H. H., Niebur, G. L. & Keaveny, T. M. Biomechanical effects of intraspecimen variations in tissue modulus for trabecular bone. J. Biomech. 35, 237–246 (2002).
Fantner, G. E. et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nature Mater. 4, 612–616 (2005).
Gao, H., Ji, B., Jager, I. L., Arzt, E. & Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl Acad. Sci. USA 100, 5597–5600 (2003).
Gupta, H. S. et al. Nanoscale deformation mechanisms in bone. Nano Lett. 5, 2108–2111 (2005).
Tai, K., Ulm, F.-J. & Ortiz, C. Nanogranular origins of the strength of bone. Nano Lett. 6, 2520–2525 (2006).
Currey, J. D. Effects of differences in mineralization on the mechanical properties of bone. Phil. Trans. R. Soc. Long B 304, 509–518 (1984).
Currey, J. D. The effect of porosity and mineral content on the Young’s modulus elasticity of compact bone. J. Biomech. 21, 131–139 (1988).
You, L. D., Weinbaum, S., Cowin, S. C. & Schaffler, M. B. Ultrastructure of the osteocyte process and its pericellular matrix. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 278, 505–513 (2004).
Tai, K., Qi, H. J. & Ortiz, C. Effect of mineral content on the nanoindentation properties and nanoscale deformation mechanisms of bovine tibial cortical bone. J. Mater. Sci.- Mater. Med. 16, 947–959 (2005).
Oliver, W. C. & Pharr, G. M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583 (1992).
Giraudguille, M. M. Twisted plywood architecture of collagen fibrils in human compact-bone osteons. Calcif. Tissue Int. 42, 167–180 (1988).
Hofmann, T., Heyroth, F., Meinhard, H., Franzel, W. & Raum, K. Assessment of composition and anisotropic elastic properties of secondary osteon lamellae. J. Biomech. 39, 2282–2294 (2006).
Kazanci, M., Roschger, P., Paschalis, E. P., Klaushofer, K. & Fratzl, P. Bone osteonal tissues by Raman spectral mapping: Orientation-composition. J. Struct. Biol. 156, 489–496 (2006).
Wagermaier, W. et al. Spiral twisting of fiber orientation inside bone lamellae. Biointerphases 1, 1–5 (2006).
Ardizzoni, A. Osteocyte lacunar size–lamellar thickness relationships in human secondary osteons. Bone 28, 215–219 (2001).
Mallat, S. A Wavelet Tour of Signal Processing (Academic, San Diego, 1998).
Donnelly, E., Baker, S. P., Boskey, A. L. & van der Meulen, M. C. H. Effects of surface roughness and maximum load on the mechanical properties of cancellous bone measured by nanoindentation. J. Biomed. Mater. Res. A 77, 426–435 (2006).
Nalla, R. K., Kinney, J. H. & Ritchie, R. O. Mechanistic fracture criteria for the failure of human cortical bone. Nature Mater. 2, 164–168 (2003).
Currey, J. D. Tensile yield in compact bone is determined by strain, post-yield behaviour by mineral content. J. Biomech. 37, 549–556 (2004).
Rho, J. Y. Ultrasonic method for measuring elastic properties of human tibial cortical and cancellous bone. Ultrasonics 34, 777–783 (1996).
Böhm, H. J. & Han, W. Comparisons between three-dimensional and two-dimensional multi-particle unit cell models for particle reinforced metal matrix composites. Modell. Simul. Mater. Sci. Eng. 9, 47–65 (2001).
Shen, H. & Lissenden, C. J. 3D finite element analysis of particle-reinforced aluminum. Mater. Sci. Eng. A 338, 271–281 (2002).
Nakamura, T. & Suresh, S. Effects of thermal residual-stresses and fiber packing on deformation of metal-matrix composites. Acta. Metal. Mater. 41, 1665–1681 (1993).
Ehrlich, P. J. & Lanyon, L. E. Mechanical strain and bone cell function: A review. Osteoporos. Int. 13, 688–700 (2002).
You, L., Cowin, S. C., Schaffler, M. B. & Weinbaum, S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 34, 1375–1386 (2001).
Burger, E. H. & Klein-Nulend, J. Mechanotransduction in bone—role of the lacuno-canalicular network. FASEB J. 13 (suppl), S101–S112 (1999).
Vesenka, J., Manne, S., Giberson, R., Marsh, T. & Henderson, E. Colloidal gold particles as an incompressible atomic force microscope imaging standard for assessing the compressibility of biomolecules. Biophys. J. 65, 992–997 (1993).
Seog, J. et al. Direct measurement of glycosamnoglycan intermolecular interactions via high-resolution force spectroscopy. Macromolecules 35, 5601–5615 (2002).
Oliver, W. C. & Pharr, G. M. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. J. Mater. Res. 19, 3–20 (2004).
Pharr, W. C. O. Indentation of an elastic planar substrate by solid. J. Mater. Res. 7, 1564 (1992).
Strang, G. & Nguyen, T. Wavelets and Filter Banks (Wellesley-Cambridge, Wellesley, MA, 1996).
Tsai, D. M. & Hsiao, B. Automatic surface inspection using wavelet reconstruction. Pattern Recognition 34, 1285–1305 (2001).
Dao, M., Chollacoop, N., Van Vliet, K. J., Venkatesh, T. A. & Suresh, S. Computational modeling of the forward and reverse problems in instrumented sharp indentation. Acta Mater. 49, 3899–3918 (2001).
Gouldstone, A. et al. Indentation across size scales and disciplines: Recent developments in experimentation and modeling. Acta Mater. 55, (2007, doi:10.1016/j.actamat.2006.08.044).
Thurner, P. et al. High-speed photography of compressed human trabecular bone correlates whitening to microscopic damage. Eng. Fracture Mech. 74, 1928–1941 (2007).
Landis, W. J. The strength of a calcified tissue depends in part on the molecular structure and organization of its constituent mineral crystals in their organic matrix. Bone 16, 533–544 (1995).
Wachtel, E. & Weiner, S. Small-angle X-ray scattering study of dispersed crystals from bone and tendon. J. Bone Miner. Res. 9, 1651–1655 (1994).
Acknowledgements
The authors thank the MIT Department of Materials Science and Engineering Nanomechanical Testing Facility, The Whitaker Foundation and the US Army through the MIT Institute for Soldier Nanotechnologies (contract number DAAD-19-02-D0002), and the NIH grant 1-R01-GM076689-01 on multiscale modelling for funding. M.D. and S.S. also acknowledge partial support from the United States Army Research Office and the Joint Improvised Explosive Devices Defeat Organization under contract number W911NF-07-1-0035. The content does not necessarily reflect the position of the government and no official endorsement should be inferred. The authors would also like to thank graphic artist Beryl Simon for preparation of Fig. 2g.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary information and supplementary figures 1-12 (PDF 662 kb)
Rights and permissions
About this article
Cite this article
Tai, K., Dao, M., Suresh, S. et al. Nanoscale heterogeneity promotes energy dissipation in bone. Nature Mater 6, 454–462 (2007). https://doi.org/10.1038/nmat1911
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1911
This article is cited by
-
Collagen piezoelectricity in osteogenesis imperfecta and its role in intrafibrillar mineralization
Communications Biology (2022)
-
Bone architecture, bone material properties, and bone turnover in non-osteoporotic post-menopausal women with fragility fracture
Osteoporosis International (2022)
-
Deconvolution of dissipative pathways for the interpretation of tapping-mode atomic force microscopy from phase-contrast
Communications Physics (2021)
-
Aggravated stress fluctuation and mechanical size effects of nanoscale lamellar bone pillars
NPG Asia Materials (2021)
-
An experimentally informed statistical elasto-plastic mineralised collagen fibre model at the micrometre and nanometre lengthscale
Scientific Reports (2021)