Abstract
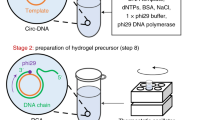
DNA is a remarkable polymer that can be manipulated by a large number of molecular tools including enzymes1. A variety of geometric objects, periodic arrays and nanoscale devices have been constructed2,3,4,5,6,7,8,9,10,11,12,13. Previously we synthesized dendrimer-like DNA and DNA nanobarcodes from branched DNA via ligases14,15. Here we report the construction of a hydrogel entirely from branched DNA that are three-dimensional and can be crosslinked in nature. These DNA hydrogels were biocompatible, biodegradable, inexpensive to fabricate and easily moulded into desired shapes and sizes. The distinct difference of the DNA hydrogel to other bio-inspired hydrogels (including peptide-based, alginate-based and DNA (linear)-polyacrylamide hydrogels16,17,18,19,20) is that the crosslinking is realized via efficient, ligase-mediated reactions. The advantage is that the gelling processes are achieved under physiological conditions and the encapsulations are accomplished in situ—drugs including proteins and even live mammalian cells can be encapsulated in the liquid phase eliminating the drug-loading step and also avoiding denaturing conditions. Fine tuning of these hydrogels is easily accomplished by adjusting the initial concentrations and types of branched DNA monomers, thus allowing the hydrogels to be tailored for specific applications such as controlled drug delivery, tissue engineering, 3D cell culture, cell transplant therapy and other biomedical applications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luo, D. The road from biology to materials. Mater. Today 6, 38–43 (2003).
Seeman, N. C. Structural DNA nanotechnology: An overview. Methods Mol. Biol. 303, 143–166 (2005).
Feldkamp, U. & Niemeyer, C. M. Rational design of DNA nanoarchitectures. Angew. Chem. Int. Edn Engl. 45, 1856–1876 (2006).
Chen, J. H. & Seeman, N. C. Synthesis from DNA of a molecule with the connectivity of a cube. Nature 350, 631–633 (1991).
Seeman, N. C. Nucleic acid junctions and lattices. J. Theor. Biol. 99, 237–247 (1982).
Kallenbach, N. R. et al. Fourth rank immobile nucleic acid junctions. J. Biomol. Struct. Dyn. 1, 159–168 (1983).
Seeman, N. C. & Kallenbach, N. R. Design of immobile nucleic acid junctions. Biophys. J. 44, 201–209 (1983).
Seeman, N. C. DNA nanotechnology: Novel DNA constructions. Ann. Rev. Biophys. Biomol. Struct. 27, 225–248 (1998).
Yan, H., Zhang, X., Shen, Z. & Seeman, N. C. A robust DNA mechanical device controlled by hybridization topology. Nature 415, 62–65 (2002).
Yan, H., Park, S. H., Finkelstein, G., Reif, J. H. & LaBean, T. H. DNA-templated self-assembly of protein arrays and highly conductive nanowires. Science 301, 1882–1884 (2003).
Seeman, N. C. From genes to machines: DNA nanomechanical devices. Trends Biochem. Sci. 30, 119–125 (2005).
Pinto, Y. Y. et al. Sequence-encoded self-assembly of multiple-nanocomponent arrays by 2D DNA scaffolding. Nano Lett. 5, 2399–2402 (2005).
Liao, S. & Seeman, N. C. Translation of DNA signals into polymer assembly instructions. Science 306, 2072–2074 (2004).
Li, Y. et al. Controlled assembly of dendrimer-like DNA. Nature Mater. 3, 38–42 (2004).
Li, Y., Cu, Y. T. & Luo, D. Multiplexed detection of pathogen DNA with DNA-based fluorescence nanobarcodes. Nature Biotechnol. 23, 885–889 (2005).
Lin, D. C., Yurke, B. & Langrana, N. A. Mechanical properties of a reversible, DNA-crosslinked polyacrylamide hydrogel. J. Biomech. Eng. 126, 104–110 (2004).
Kong, H. J., Kaigler, D., Kim, K. & Mooney, D. J. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules 5, 1720–1727 (2004).
Luo, Y. & Shoichet, M. S. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nature Mater. 3, 249–253 (2004).
Kisiday, J. et al. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: implications for cartilage tissue repair. Proc. Natl Acad. Sci. USA 99, 9996–10001 (2002).
Holmes, T. C. et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc. Natl Acad. Sci. USA 97, 6728–6733 (2000).
Yang, D., Strode, J. T., Spielmann, H. P., Wang, A. H.-J. & Burke, T. G. DNA interactions of two clinical camptothecin drugs stabilize their active lactone forms. J. Am. Chem. Soc. 120, 2979–2980 (1998).
Wooldridge, J. E. & Weiner, G. J. CpG DNA and cancer immunotherapy: Orchestrating the antitumor immune response. Curr. Opin. Oncol. 15, 440–445 (2003).
Dalpke, A. H. & Heeg, K. CpG-DNA as immune response modifier. Int. J. Med. Microbiol. 294, 345–354 (2004).
Um, S., Lee, J., Kwon, S., Li, Y. & Luo, D. Dendrimer-like DNA (DL-DNA) based fluorescence nanobarcodes. Nature Protocols 1, 995–1000 (2006).
Luo, D., Woodrow-Mumford, K., Belcheva, N. & Saltzman, W. M. Controlled DNA delivery systems. Pharm. Res. 16, 1300–1308 (1999).
Acknowledgements
We wish to acknowledge financial support from USDA, the Cornell Advanced Technology Centre for Biotechnology, the Nanobiotechnology Center (NSF/ECS-9876771) and the Cornell Center for Materials Research (NSF/DMR-0520404). D.L. acknowledges the NSF CAREER Award and N.P. acknowledges the Korea Research Foundation Grant (04-03-05-2) for partial support. Y. Chang, Y. Zhang and N. Walker are acknowledged for help in animal tests, DMA and data processing, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The corresponding author (D.L.) is one of the founders of a start-up company that licensed the technology from Cornell University.
Supplementary information
Supplementary Information
Supplementary tables S1, S2 and S3
Supplementary Information
Supplementary information and figures S1, S2 and S3
Rights and permissions
About this article
Cite this article
Um, S., Lee, J., Park, N. et al. Enzyme-catalysed assembly of DNA hydrogel. Nature Mater 5, 797–801 (2006). https://doi.org/10.1038/nmat1741
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1741
This article is cited by
-
Cell unit-inspired natural nano-based biomaterials as versatile building blocks for bone/cartilage regeneration
Journal of Nanobiotechnology (2023)
-
Dynamic matrices with DNA-encoded viscoelasticity for cell and organoid culture
Nature Nanotechnology (2023)
-
Vacuole dynamics and popping-based motility in liquid droplets of DNA
Nature Communications (2023)
-
A temperature-sensitive DNA-PNIPAAm hydrogel prepared by base pairing
Colloid and Polymer Science (2023)
-
Preparation of Photo-responsive DNA Supramolecular Hydrogels and Their Application as UV Radiometers
Chemical Research in Chinese Universities (2023)