Abstract
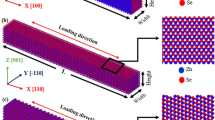
Nanometre-sized inorganic dots, wires and belts have a wide range of electrical and optical properties, and variable mechanical stability and phase-transition mechanisms that show a sensitive dependency on size, shape and structure. The optical properties of the semiconductor ZnS in wurtzite structures are considerably enhanced, but the lack of structural stability limits technological applications. Here, we demonstrate that morphology-tuned wurtzite ZnS nanobelts show a particular low-energy surface structure dominated by the  surface facets. Experiments and calculations show that the morphology of ZnS nanobelts leads to a very high mechanical stability to ∼6.8 GPa, and also results in an explosive mechanism for the wurtzite-to-sphalerite phase transformation together with in situ fracture of the nanobelts. ZnS wurtzite nanobelts provide a model that is useful not only for understanding the morphology-tuned stability and transformation mechanism, but also for improving synthesis of metastable nanobelts with quantum effects for electronic and optical devices.
surface facets. Experiments and calculations show that the morphology of ZnS nanobelts leads to a very high mechanical stability to ∼6.8 GPa, and also results in an explosive mechanism for the wurtzite-to-sphalerite phase transformation together with in situ fracture of the nanobelts. ZnS wurtzite nanobelts provide a model that is useful not only for understanding the morphology-tuned stability and transformation mechanism, but also for improving synthesis of metastable nanobelts with quantum effects for electronic and optical devices.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Monroy, E., Omnes, F. & Calle, F. Wide-band gap semiconductor ultraviolet photodetectors. Semicond. Sci. Technol. 18, R33–R51 (2003).
Bhargava, R. N., Gallagher, D., Hong, X. & Nurmikko, D. Optical properties of manganese-doped nanocrystals of ZnS. Phys. Rev. Lett. 72, 416–419 (1994).
Park, W., King, J. S., Neff, C. W., Liddell, C. & Summers, C. ZnS-based photonic crystals. Phys. Status Solidi b 229, 949–960 (2002).
Gilbert, B. et al. X-ray absorption spectroscopy of the cubic and hexagonal polytypes of zinc sulfide. Phys. Rev. B 66, 245205 (2002).
Qadri, S. B. et al. The effect of particle size on the structural transitions in zinc sulfide. J. Appl. Phys. 89, 115–119 (2001).
Desgreniers, S., Beaulieu, L. & Lepage, I. Pressure induced structural changes in ZnS. Phys. Rev. B 61, 8726–8733 (2000).
Qadri, S. B. et al. Size-induced transition-temperature reduction in nanoparticles of ZnS. Phys. Rev. B 60, 9191–9193 (1999).
Alivisatos, A. P. Semiconductor clusters, nanocrystals, and quantum dots. Science 271, 933–937 (1996).
Lieber, C. M. One-dimensional nanostructures: chemistry, physics applications. Solid State Commun. 107, 607–616 (1998).
Zhao, Y. W. et al. Low-temperature synthesis of hexagonal (wurtzite) ZnS nanocrystals. J. Am. Chem. Soc. 126, 6874–6875 (2004).
Ma, C., Moore, D., Li, J. & Wang, Z. L. Nanobelts, nanocombs, and nanowindmills of wurtzite ZnS. Adv. Mater. 15, 228–231 (2003).
Wang, Z. W. et al. A quenchable superhard carbon phase synthesized by cold compression of carbon nanotubes. Proc. Natl Acad. Sci. USA 101, 13699–13702 (2004).
Zaziski, D. et al. Critical size for fracture during solid-solid phase transformations. Nano Lett. 4, 943–946 (2004).
Chen, C. C., Herhold, A. B., Johnson, C. S. & Alivisatos, A. P. Size dependence of structural metastability in semiconductor nanocrystals. Science 276, 398–401 (1997).
Tolbert, S. H. & Alivisatos, A. P. The wurtzite to rock salt structural transformation in CdSe nanocrystals under high pressure. J. Chem. Phys. 102, 4642–4656 (1995).
Barin, I., Knacke, O. & Kubschewski, O. Thermochemical Properties of Inorganic Substances 827–828 (Springer, Berlin, 1977).
Hamad, S., Cristol, S. & Catlow, C. R. Surface structures and crystal morphology of ZnS: Computational study. J. Phys. Chem. B 106, 11002–11008 (2002).
Wright, K., Watson, G. W., Parker, S. C. & Vaughan, D. J. Simulation of the structure and stability of sphalerite (ZnS) surfaces. Am. Mineral. 83, 141–146 (1998).
Wright, K. & Gale, J. D. Interatomic potentials for the simulation of the zinc-blende and wurtzite forms of ZnS and CdS: Bulk structure, properties, and phase stability. Phys. Rev. B 70, 035211 (2004).
Zhang, H. Z., Huang, F., Gilbert, B. & Banfield, J. F. Molecular dynamics simulations, thermodynamic analysis, and experimental study of phase stability of zinc sulfide nanoparticles. J. Phys. Chem. B 107, 13051–13060 (2003).
Wang, Z. L. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J. Phys. Chem. B 104, 1153–1175 (2000).
Williams, V. A. Diffusion of some impurities in zinc sulfide single crystals. J. Mater. Sci. 7, 807–812 (1972).
Birman, J. L. Simplified LCAO method for zincblende, wurtzite, and mixed crystal structures. Phys. Rev. 115, 1493–1905 (1959).
Posfai, M. & Buseck, P. R. in Modular Aspects of Minerals Vol. 1 (ed. Merlino, S.) 193–235 (EMU Notes in Mineralogy, Eötvös Univ. Press, Budapest, 1997).
Alivisatos, P. The use of nanocrystals in biological detection. Nature Biotechnol. 22, 47–52 (2004).
Ding, Y., Wang, X. D. & Wang, Z. L. Phase controlled synthesis of ZnS nanobelts: zinc blende vs wurtzite. Chem. Phys. Lett. 398, 32–36 (2004).
Acknowledgements
We appreciate financial support from the Director’s Funded Postdoctoral Fellowship at Los Alamos National Laboratory. We also acknowledge gratefully the staff at CHESS, Wilson Laboratory of Cornell University for assistance with experimental matters. X.D.W. and Z.L.W. are grateful for support from NSF. Special appreciation goes to the Carnegie/DOE Alliance Center (CDAC) for significant support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wang, Z., Daemen, L., Zhao, Y. et al. Morphology-tuned wurtzite-type ZnS nanobelts. Nature Mater 4, 922–927 (2005). https://doi.org/10.1038/nmat1522
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1522
This article is cited by
-
A Paradigm Shift in High-Pressure Equation of State Modeling: Unveiling the Pressure–Bulk Modulus Relationship
Iranian Journal of Science (2023)
-
Induced structural modifications in ZnS nanowires via physical state of catalyst: Highlights of 15R crystal phase
Nano Research (2022)
-
Crystal and electronic facet analysis of ultrafine Ni2P particles by solid-state NMR nanocrystallography
Nature Communications (2021)
-
Facile synthesis of ZnS/MnS nanocomposites for supercapacitor applications
Journal of Solid State Electrochemistry (2018)
-
Electrical properties and aquatic ecotoxicity effects of ZnS nanocrystals
Electrical Engineering (2018)