Abstract
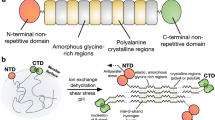
Spider silks are some of the strongest materials found in nature1,2. Achieving the high tensile strength and elasticity of the dragline of orb-weaving spiders, such as Nephila clavipes3,4,5, is a principal goal in biomimetics research. The dragline has a composite nature and is predominantly made up by two proteins, the major ampullate spidroins 1 and 2 (refs 37), which can be considered natural block copolymers8. On the basis of their molecular structures both spidroins are thought to contribute, in different ways, to the mechanical properties of dragline silk9. The spinning process itself is also considered important for determining the observed features by shaping the hierarchical structure of the fibre10,11. Here we study the heterogeneous distribution of proteins along the radial axis of the fibre. This heterogeneity is generated during the conversion of the liquid spinning dope into solid fibre. Whereas spidroin 1 is distributed almost uniformly within the fibre core, spidroin 2 is missing in the periphery and is tightly packed in certain core areas. Our findings suggest that the role of spidroin 2 in the spinning process could be to facilitate the formation of fibrils and contribute directly to the elasticity of the silk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hinman, M., Dong, Z., Xu, M. & Lewis, R. V. in Biopolymers (ed. Case, S. T.) 227–254 (Springer, Berlin, 1992).
Hinman, M. B., Jones, J. A. & Lewis, R. V. Synthetic spider silk: a modular fiber. Trends Biotechnol. 18, 374–379 (2000).
Guerette, P. A., Ginzinger, D. G., Weber, B. H. & Gosline, J. M. Silk properties determined by gland-specific expression of a spider fibroin gene family. Science 272, 112–115 (1996).
Thiel, B. & Viney, C. A nonperiodic lattice model for crystals in Nephila clavipes major ampullate silk. Mater. Res. Soc. Bull. 20, 52–56 (1995).
Hayashi, C. Y. & Lewis, R. V. Molecular architecture and evolution of a modular spider silk protein gene. Science 287, 1477–1479 (2000).
Xu, M. & Lewis, R. V. Structure of a protein superfiber: spider dragline silk. Proc. Natl Acad. Sci. USA 87, 7120–7124 (1990).
Hinman, M. B. & Lewis, R. V. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J. Biol. Chem. 267, 19320–19324 (1992).
Jelinski, L. W. et al. Orientation, structure, wet-spinning, and molecular basis for supercontraction of spider dragline silk. Int. J. Biol. Macromol. 24, 197–201 (1999).
Hayashi, C. Y., Shipley, N. H. & Lewis, R. V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 24, 271–275 (1999).
Vollrath, F. Strength and structure of spiders’ silks. J. Biotechnol. 74, 67–83 (2000).
Vollrath, F. & Knight, D. P. Liquid crystalline spinning of spider silk. Nature 410, 541–548 (2001).
Kaplan, D. L., Lombardi, S. J., Muller, W. S. & Fossey, S. A. in Biomaterial - Novel Materials from Biological Sources 1 (ed. Byrom, D.) (Macmillan and ICI Biological Products Business, New York, 1991).
Sponner, A. et al. Characterization of the protein components of Nephila clavipes dragline silk. Biochemistry 44, 4727–4736 (2005).
Knight, D. P. & Vollrath, F. Changes in element composition along the spinning duct in a Nephila spider. Naturwissenschaften 88, 179–182 (2001).
Tillinghast, E. K., Chase, S. F. & Townley, M. A. Water extraction by the major ampullate duct during silk formation in the spider Agriope Aurantia Lucas. J. Insect Physiol. 30, 591–596 (1984).
Kovoor, J. & Zylberberg, L. Morphologie et ultrastructure du canal des glandes ampullac,es d’Araneus diadematus Clerk (Arachnida, Araneidae). Z. Zellforsch. Mikrosk. Anat. 128, 188–211 (1972).
Vollrath, F., Knight, D. P. & Hu, X. W. Silk production in a spider involves acid bath treatment. Proc. R. Soc. Lond. B 265, 817–820 (1998).
Willcox, J. P., Gido, S. P., Muller, W. & Kaplan, D. Evidence of a cholesteric liquid crystalline phase in natural silk spinning process. Macromolecules 29, 5106–5110 (1996).
Kerkham, K., Viney, C., Kaplan, D. & Lombardi, S. Liquid crystallinity of natural silk secretion. Nature 349, 596–598 (1991).
Work, R. W. Duality in major ampullate silk and precursive material from orb-web-building spiders (Araneae). Trans. Am. Microsc. Soc. 103, 113–121 (1984).
Frische, S., Maunsbach, A. B. & Vollrath, F. Elongate cavities and skin-core structure in Nephila spider silk observed by electron microscopy. J. Microsc. 189, 64–70 (1998).
Li, S. F., McGhie, A. J. & Tang, S. L. New internal structure of spider dragline silk revealed by atomic force microscopy. Biophys. J. 66, 1209–1212 (1994).
Bell, A. L. & Peakall, D. B. Changes in fine structure during silk protein production in the ampullate gland of the spider Araneus sericatus. J. Cell Biol. 42, 284–295 (1969).
Knight, D. P., Knight, M. M. & Vollrath, F. Beta transition and stress-induced phase separation in the spinning of spider dragline silk. Int. J. Biol. Macromol. 27, 205–210 (2000).
Thiel, B. L., Guess, K. B. & Viney, C. Spider major ampullate silk (drag line): smart composite processing based on imperfect crystals. J. Microsc. 185, 179–187 (1997).
Thiel, B. L., Guess, K. B. & Viney, C. Non-periodic lattice crystals in the hierarchical microstructure of spider (major ampullate) silk. Biopolymers 41, 703–719 (1997).
Bendayan, M. in Colloidal Gold Post-Embedding Immunocytochemistry (ed. Graumann, W.) (Gustav Fischer, Stuttgart, 1995).
Oroudjev, E. et al. Segmented nanofibers of spider dragline silk: Atomic force microscopy and single-molecule force spectroscopy. Proc. Natl Acad. Sci. USA 99, 6460–6465 (2002).
Augsten, K., Muehlig, P. & Hermann, C. Glycoproteins and skin-core structure in Nephila clavipes spider silk observed by light and electron microscopy. Scanning 22, 12–15 (2000).
Riekel, C., Rossle, M., Sapede, D. & Vollrath, F. Influence of CO2 on the micro-structural properties of spider dragline silk: X-ray microdiffraction results. Naturwissenschaften 91, 30–33 (2004).
Shao, Z. Z., Hu, X. W., Frische, S. & Vollrath, F. Heterogeneous morphology of Nephila edulis spider silk and its significance for mechanical properties. Polymer 40, 4709–4711 (1999).
Porter, D., Vollrath, F. & Shao, Z. Predicting the mechanical properties of spider silk as a model nanostructured polymer. Eur. Phys. J. E S. 16, 199–206 (2005).
Acknowledgements
The authors want to thank U. Stephan and K. Hartung for excellent technical assistance. This work was supported by Bundesministerium für Forschung und Bildung (BMBF FKZ 0311130), Bundesministerium für Landwirtschaft (BML FKZ 98NR049) and Thueringer Ministerium für Wissenschaft, Forschung und Kultur (TMWFK B307-99-001). A. Sponner was supported by an EU TOK grant (MTKD-CT-2004-014533).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sponner, A., Unger, E., Grosse, F. et al. Differential polymerization of the two main protein components of dragline silk during fibre spinning. Nature Mater 4, 772–775 (2005). https://doi.org/10.1038/nmat1493
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1493
This article is cited by
-
Regionalization of cell types in silk glands of Larinioides sclopetarius suggest that spider silk fibers are complex layered structures
Scientific Reports (2023)
-
Spidroins and Silk Fibers of Aquatic Spiders
Scientific Reports (2019)
-
Micromechanics of fresh and 30-year-old Nephila inaurata madagascariensis dragline silk
Journal of Materials Science (2017)
-
Scrutinizing the datasets obtained from nanoscale features of spider silk fibres
Scientific Data (2014)
-
Unravelling the biodiversity of nanoscale signatures of spider silk fibres
Nature Communications (2013)