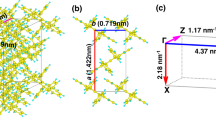
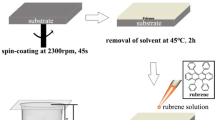
Abstract
Electronic devices based on single crystals of organic semiconductors provide powerful means for studying intrinsic charge-transport phenomena and their fundamental electronic limits1,2,3,4. However, for technological exploitation, it is imperative not to be confined to the tedious growth and cumbersome manipulation of molecular crystals—which generally show notoriously poor mechanical properties—but to be able to process such materials into robust architectures by simple and efficient means. Here, we advance a general route for facile fabrication of thin-film devices from solution. The key beneficial feature of our process—and the principal difference from existing vapour deposition5,6,7 and solution-processing schemes7,8,9,10—is the incorporation of a glass-inducing diluent that enables controlled crystallization from an initial vitreous state of the organic semiconductor, formed in a selected area of the phase diagram of the two constituents. We find that the vitrifying diluent does not adversely affect device performance. Indeed, our environmentally stable, discrete rubrene-based transistors rival amorphous silicon devices, reaching saturated mobilities of up to 0.7 cm2 V−1 s−1, ON–OFF ratios of ≥106 and subthreshold slopes as steep as 0.5 V per decade. A nearly temperature-independent device mobility, indicative of a high crystalline quality of our solution-processed, rubrene-based films11, corroborates these findings. Inverter and ring-oscillator structures are also demonstrated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Podzorov, V., Pudalov, V. M. & Gershenson, M. E. Field-effect transistors on rubrene single crystals with parylene gate insulator. Appl. Phys. Lett. 82, 1739–1741 (2003).
Podzorov, V., Sysoev, S. E., Loginova, E., Pudalov, V. M. & Gershenson, M. E. Single-crystal organic field effect transistors with the hole mobility ∼8 cm2/V s. Appl. Phys. Lett. 83, 3504–3505 (2003).
Sundar, V. C. et al. Elastomeric transistor stamps: Reversible probing of charge transport in organic crystals. Science 303, 1644–1646 (2004).
de Boer, R. W. I., Klapwijk, T. M. & Morpurgo, A. F. Field-effect transistors on tetracene single crystals. Appl. Phys. Lett. 83, 4345–4347 (2003).
Klauk, H., Gundlach, D. J., Nichols, J. A. & Jackson, T. N. Pentacene organic thin-film transistors for circuit and display applications. IEEE Trans. Electron Dev. 46, 1258–1262 (1999).
Dimitrakopoulos, C. D., Purushothaman, S., Kymissis, J., Callegari, A. & Shaw, J. M. Low-voltage organic transistors on plastic comprising high-dielectric constant gate insulators. Science 284, 822–824 (1999).
Dimitrakopoulos, C. D. & Malenfant, P. R. L. Organic thin film transistors for large area electronics. Adv. Mater. 14, 99–117 (2002).
Sirringhaus, H. et al. Two-dimensional charge transport in self-organised, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Afzali, A., Dimitrakopoulos, C. D. & Breen, T. L. High-performance, solution-processed organic thin film transistors from a novel pentacene precursor. J. Am. Chem. Soc. 124, 8812–8813 (2002).
Anthony, J. E., Brooks, J. S., Eaton, D. L. & Parkin, S. R. Functionalized pentacene: Improved electronic properties from control of solid-state order. J. Am. Chem. Soc. 123, 9482–9483 (2001).
Nelson, S. F., Lin, Y. Y., Gundlach, D. J. & Jackson, T. N. Temperature-independent transport in high-mobility pentacene transistors. Appl. Phys. Lett. 72, 1854–1856 (1998).
Herwig, P. T. & Müllen, K. A soluble pentacene precursor: Synthesis, solid-state conversion into pentacene and application in a field-effect transistor. Adv. Mater. 11, 480–483 (1999).
Kagan, C. R., Mitzi, D. B. & Dimitrakopoulos, C. D. Organic–inorganic hybrid materials as semiconducting channels in thin-film field-effect transistors. Science 286, 945–947 (1999).
Mitzi, D. B., Kosbar, L. L., Murray, C. E., Copel, M. & Afzali, A. High-mobility ultrathin semiconducting films prepared by spin coating. Nature 428, 299–303 (2004).
Katz, H. E. et al. Mesophase transitions, surface functionalization, and growth mechanism of semiconducting 6PTTP6 films from solution. J. Phys. Chem. B 108, 8567–8571 (2004).
Chabinyc, M. L., Wong, W. S., Paul, K. E. & Street, R. A. Fabrication of arrays of organic polymeric thin-film transistors using self-aligned microfluidic channels. Adv. Mater. 15, 1903–1907 (2003).
Pope, M. & Swenberg, C. E. Electronic Processes in Organic Crystals and Polymers (Oxford Univ. Press, New York, 1982).
Carpay, F. M. A. & Cense, W. A. Production of in situ composites by unidirectional crystalline decomposition of non-crystalline solids. Nat. Phys. Sci. 241, 19–20 (1973).
Henn, D. E., Williams, W. G. & Gibbons, D. J. Crystallographic data for an orthorhombic form of rubrene. J. Appl. Crystallogr. 4, 256 (1971).
Vrijmoeth, J., Stok, R. W., Veldman, R. M., Schoonveld, W. A. & Klapwijk, T. M. Single crystallites in “planar polycrystalline” oligothiophene films: Determination of orientation and thickness by polarization microscopy. J. Appl. Phys. 83, 3816–3824 (1998).
Kepler, R. G. in Organic Semiconductors (eds Brophy, J. & Buttrey, J. W.) 1–20 (Macmillan, New York, 1962).
Bakhuis Roozeboom, H.W. Erstarrungspunkte der Mischkrystalle zweier Stoffe. Z. Phys. Chem. 30, 385–412 (1899).
Meijer, E. J. et al. Dopant density determination in disordered organic field-effect transistors. J. Appl. Phys. 93, 4831–4835 (2003).
Bakhuis Roozeboom, H. W. Die Heterogenen Gleichgewichte vom Standpunkte der Phasenlehre (Vieweg, Braunschweig, 1901).
Gelinck, G. H. et al. Flexible active-matrix displays and shift registers based on solution-processed organic transistors. Nature Mater. 3, 106–110 (2004).
Acknowledgements
We are indebted to our colleagues at Philips, S. Setayesh and E. Cantatore, for discussions. K. Pernstich and D. Gundlach (ETH Zürich) provided invaluable and highly critical data regarding vacuum-evaporated rubrene devices, for which we are deeply grateful. M. Beenhakkers (Philips) prepared the test devices for the present work. C.T. would like to thank the DPI for financial support. Furthermore, the EUROSCORES SONS is acknowledged for support. N.S. is very grateful to the Swiss Federal Office for Education and Science for a post-doctoral fellowship in the framework of the EU research programme IHP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Stingelin-Stutzmann, N., Smits, E., Wondergem, H. et al. Organic thin-film electronics from vitreous solution-processed rubrene hypereutectics. Nature Mater 4, 601–606 (2005). https://doi.org/10.1038/nmat1426
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1426
This article is cited by
-
Enabling three-dimensional porous architectures via carbonyl functionalization and molecular-specific organic-SERS platforms
Nature Communications (2021)
-
Research progress of rubrene as an excellent multifunctional organic semiconductor
Frontiers of Physics (2021)
-
Morphology and transport characterization of solution-processed rubrene thin films on polymer-modified substrates
Scientific Reports (2020)
-
Water stable molecular n-doping produces organic electrochemical transistors with high transconductance and record stability
Nature Communications (2020)
-
Ultrasonication-Mediated Self-Assembly in Polythiophene Films via Control of Residual Solvent Evaporation
Macromolecular Research (2018)