Abstract
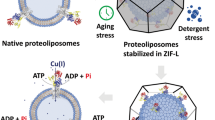
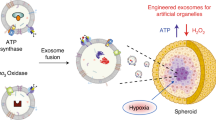
Sol–gel immobilization of soluble proteins has proven to be a viable method for stabilizing a wide variety of proteins in transparent inorganic matrices1,2,3. The encapsulation of membrane-bound proteins has received much less attention, although work in this area suggests potential opportunities in microarray technology and high-throughput drug screening4,5. The present paper describes a liposome/sol–gel architecture in which the liposome provides membrane structure and protein orientation to two transmembrane proteins, bacteriorhodopsin (bR) and F0F1-ATP synthase; the sol–gel encapsulation converts the liposomal solution into a robust material without compromising the intrinsic activity of the incorporated proteins. Here we report on two different proteoliposome-doped gels (proteogels) whose properties are determined by the transmembrane proteins. Proteogels containing bR proteoliposomes exhibit a stable proton gradient when irradiated with visible light, whereas proteogels containing proteoliposomes with both bR and F0F1-ATP synthase couple the photo-induced proton gradient to the production of ATP. These results demonstrate that materials based on the liposome/sol–gel architecture are able to harness the properties of transmembrane proteins and enable a variety of applications, from power generation and energy storage to the powering of molecular motors, and represent a new technology for performing complex chemical synthesis in a solid-state matrix.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Avnir, D., Braun, S., Lev, O. & Ottolenghi, M. Enzymes and other proteins entrapped in sol–gel materials. Chem. Mater. 6, 1605–1614 (1994).
Zink, J. I., Valentine, J. S. & Dunn, B. Biomolecular materials based on sol–gel encapsulated proteins. New J. Chem. 18, 1109–1115 (1994).
Livage, J., Coradin, T. & Roux, C. Encapsulation of biomolecules in silica gels. J. Phys. Condens. Matter. 13, 673–691 (2001).
Besanger, T. R. & Brennan, J. D. Ion sensing and inhibition studies using the transmembrane ion channel peptide gramicidin a entrapped in sol–gel-derived silica. Anal. Chem. 75, 1094–1101 (2003).
Besanger, T. R., Easwaramoorthy, B. & Brennan, J. D. Entrapment of highly active membrane-bound receptors in macroporous sol–gel derived silica. Anal. Chem. 76, 6470–6475 (2004).
Yamanaka, S. A., Charych, D. H., Loy, D. A. & Sasaki, D. Y. Solid phase immobilization of optically responsive liposomes in sol–gel materials for chemical and biological sensing. Langmuir 13, 5049–5053 (1997).
Nassif, N. et al. Living bacteria in silica gels. Nature Mater. 1, 42–44 (2002).
Carturan, G., Dal Toso, R., Boninsegna, S. & Dal Monte, R. Encapsulation of functional cells by sol–gel silica: actual progress and perspectives for cell therapy. J. Mater. Chem. 14, 2087–2098 (2004).
Wu, S. et al. Bacteriorhodopsin encapsulated in transparent sol–gel glass: A new biomaterial. Chem. Mater. 5, 115–120 (1993).
Oesterhelt, D. & Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nature New Biol. 233, 149–152 (1971).
Oesterhelt, D. & Stoeckenius, W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 31, 667–678 (1974).
Oesterhelt, D. & Schuhmann, L. Reconsitituion of bacteriorhodopsin. FEBS Lett. 44, 262–265 (1974).
Birge, R. R. Photophysics and molecular electronic application of the rhodopsins. Annu. Rev. Phys. Chem. 41, 683–733 (1990).
Birge, R. R. Nature of the primary photochemical events in rhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. 1016, 293–327 (1990).
Rigaud, J. L., Bluzat, A. & Buschlen, S. Incorporation of bacteriorhodopsin into large unilamellar liposomes by reverse phase evaporation. Biochem. Biophys. Res. Com. 111, 373–382 (1983).
Seigneuret, M. & Rigaud, J. L. Use of fluorescent pH probe pyranine to detect heterogeneous directions of proton movement in bacteriorhodopsin reconsituted large liposomes. FEBS Lett. 188, 101–106 (1985).
Szoka, F. & Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl Acad. Sci. USA 75, 4194–4198 (1978).
Straubinger, R. M., Papahadjopoulos, D. & Hong, K. Endocytosis and intracellular fate of liposomes using pyranine as probe. Biochemistry 29, 4929–4939 (1990).
Seigneuret, M. & Rigaud, J. L. Analysis of passive and light-driven ion movements in large bacteriorhodopsin liposomes reconstituted by reverse-phase evaporation. 1. Factors governing the passive proton permeability of the membrane. Biochem. 25, 6716–6722 (1986).
Ferrer, M. L., Monte, F. D. & Levy, D. A novel and simple alcohol-free sol–gel route for encapsulation of labile proteins. Chem. Mater. 14, 3619–3621 (2002).
Dunn, B. & Zink, J. I. Probes of pore environment and molecule-matrix interfactions in sol–gel materials. Chem. Mater. 9, 2280–2291 (1997).
Matsuda, A. et al. in Sol–gel Optics (eds Mackenzie, J. D. & Ulrich, D. R.) 62–70 (SPIE, Bellingham, 1990).
Chen, Q., Kenausis, G. L. & Heller, A. Stability of oxidases immobilized in silica gel. J. Am. Chem. Soc. 120, 4582–4585 (1998).
Nguyen, D. T., Smit, M., Dunn, B. & Zink, J. I. Stabilization of creatine kinase encapsulated in silicate sol–gel materials and unusual temperature effects on its activity. Chem. Mater. 14, 4300–4306 (2002).
Liu, H. Q. et al. Control of a biomolecular motor-powered nanodevice with an engineered chemical switch. Nature Mater. 1, 173–177 (2002).
Clemmens, J. et al. Motor-protein “roundabouts”: Microtubules moving on kinesin-coated tracks through engineered networks. Lab on a Chip 4, 83–86 (2004).
Dencher, N. A. & Heyn, M. P. Bacteriorhodopsin monomers pump protons. FEBS Lett. 108, 307–10 (1979).
Hazard, A. & Montemagno, C. Improved purification for thermophilic F1F0 ATP synthase using n-dodecyl beta-D-maltoside. Arch. Biochem. Biophys. 407, 117–124 (2002).
Ozogul, F., Taylor, K. D. A., Quantick, P. C. & Ozogul, Y. A rapid HPLC-determination of ATP-related compounds and its application to herring stored under modified atmosphere. Int. J. Food Sci. Technol. 35, 549–554 (2000).
Acknowledgements
This work was also partially supported by Center for Cell Mimetic Space Exploration (CMISE), a NASA University Research, Engineering and Technology Institute (URETI), under award number NCC 2-1364. Additional funding was obtained from the National Science Foundation (DMR 0103952 and DMR 0099862).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Luo, TJ., Soong, R., Lan, E. et al. Photo-induced proton gradients and ATP biosynthesis produced by vesicles encapsulated in a silica matrix. Nature Mater 4, 220–224 (2005). https://doi.org/10.1038/nmat1322
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1322
This article is cited by
-
Reconstitution of FoF1-ATPase-based biomimetic systems
Nature Reviews Chemistry (2019)
-
Formation of self-aggregated and interconnected silver network within sol–gel silica
Journal of Materials Science (2013)
-
Chlorosome antenna complexes from green photosynthetic bacteria
Photosynthesis Research (2013)
-
Modifiying glassy carbon electrode with ferrocene-bridged polysilsesquioxanes
Journal of Sol-Gel Science and Technology (2010)