Abstract
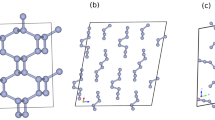
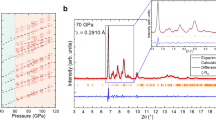
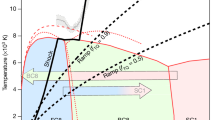
Nitrogen usually consists of molecules where two atoms are strongly triple-bonded. Here, we report on an allotropic form of nitrogen where all atoms are connected with single covalent bonds, similar to carbon atoms in diamond. The compound was synthesized directly from molecular nitrogen at temperatures above 2,000 K and pressures above 110 GPa using a laser-heated diamond cell. From X-ray and Raman scattering we have identified this as the long-sought-after polymeric nitrogen with the theoretically predicted cubic gauche structure (cg-N). This cubic phase has not been observed previously in any element. The phase is a stiff substance with bulk modulus ≥300 GPa, characteristic of strong covalent solids. The polymeric nitrogen is metastable, and contrasts with previously reported amorphous non-molecular nitrogen, which is most likely a mixture of small clusters of non-molecular phases. The cg-N represents a new class of single-bonded nitrogen materials with unique properties such as energy capacity: more than five times that of the most powerfully energetic materials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Greenwood, N.N. & Earnshaw, A. Chemistry of the Elements (Pergamon, Oxford, 1984).
McMahan, A.K. & LeSar, R. Pressure dissociation of solid nitrogen under 1 Mbar. Phys. Rev. Lett. 54, 1929–1932 (1985).
Mailhiot, C., Yang, L.H. & McMahan, A.K. Polymeric nitrogen. Phys. Rev. B 46, 14419–14435 (1992).
Martin, R.M. & Needs, R. Theoretical study of the molecular-to-nonmolecular transformation of nitrogen at high pressures. Phys. Rev. B 34, 5082–5092 (1986).
Barbee III, T.W. Metastability of atomic phases of nitrogen. Phys. Rev. B 48, 9327–9330 (1993).
Mitas, L. & Martin, R.M. Quantum Monte Carlo of nitrogen: atom, dimer, atomic, and molecular solids. Phys. Rev. Lett. 72, 2438–2441 (1994).
Alemany, M.M.G. & Martins, J.L. Density-functional study of nonmolecular phases of nitrogen: Metastable phase at low pressure. Phys. Rev. B 68, 024110 (2003).
Yakub, E.S. Diatomic fluids at high pressures and temperatures: a non-empirical approach. Physica B 265, 31–38 (1999).
Mattson, W.D. The Complex Behavior of Nitrogen Under Pressure: Ab Initio Simulation of the Properties of Sructure and Shock Waves p. 108, Thesis, Univ. Illinois at Urbana-Champaign (2003).
Eremets, M.I., Hemley, R.J., Mao, H.K. & Gregoryanz, E. Semiconducting non-molecular nitrogen up to 240 GPa and its low-pressure stability. Nature 411, 170–174 (2001).
Goncharov, A.F., Gregoryanz, E., Mao, H.K., Liu, Z. & Hemley, R.J. Optical evidence for nonmolecular phase of nitrogen above 150 GPa. Phys. Rev. Lett. 85, 1262–1265 (2000).
Gregoryanz, E., Goncharov, A.F., Hemley, R.J. & Mao, H.K. High-pressure amorphous nitrogen. Phys. Rev. B 64, 052103 (2001).
Gregoryanz, E. et al. Raman, infrared, and x-ray evidence for new phases of nitrogen at high pressures and temperatures. Phys. Rev. B 66, 224108 (2002).
Boehler, R., von Bargen, N. & Chopelas, A. Melting, thermal expansion, and phase transitions of iron at high pressures. J. Geophys. Res. B 95, 21731–21736 (1990).
Eremets, M.I., Struzhkin, V.V., Mao, H.K. & Hemley, R.J. Superconductivity in boron. Science 293, 272–274 (2001).
Jephcoat, A.P., Hemley, R.J., Mao, H.K. & Cox, D.E. Pressure-induced structural transitions in solid nitrogen. Bull. Am. Phys. Soc. 33, 522 (1988).
Olijnyk, H. High pressure x-ray diffraction studies on solid N2 up to 43.9 GPa. J. Chem. Phys. 93, 8968–8972 (1990).
Hanfland, M., Lorenzen, M., Wassilew-Reul, C. & Zontone, F. Structures of molecular nitrogen at high pressure. Rev. High Press. Sci. Technol. 7, 787–789 (1998).
Sanz, D.N., Loubeyre, P. & Mezouar, M. Equation of state and pressure induced amorphization of β-boron from X-ray measurements up to 100 GPa. Phys. Rev. Lett. 89, 245501 (2002).
Yoo, C.S., Akella, J., Cynn, H. & Nicol, M. Direct elementary reactions of boron and nitrogen at high pressures and temperatures. Phys. Rev. B 56, 140–146 (1997).
Mao, H.K., Xu, J. & Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasihydrostatic conditions. J. Geophys. Res. 91, 4673–4676 (1986).
Hanfland, M. & Syassen, K. A Raman study of diamond anvils under stress. J. Appl. Phys. 57, 2752–2756 (1984).
Boppart, H., von Straaten, J. & Silvera, I. Raman scattering of diamond at high pressures. Phys. Rev. B 32, 1423–1425 (1985).
Eremets, M.I. Megabar high-pressure cells for Raman measurements. J. Raman Spectrosc. 34, 515–518 (2003).
Eremets, M.I. High Pressure Experimental Methods (Oxford Univ. Press, Oxford, 1996).
Knittle, E., Wentzcovitch, R.M., Jeanloz, R. & Cohen, M.L. Experimental and theoretical equation of state of cubic boron nitride. Nature 337, 349–352 (1989).
Gregoryanz, E. et al. Synthesis and characterization of a binary noble metal nitride. Nature Mater. 3, 294–297 (2004).
Acknowledgements
We are thankful to M. Hanfland for his help with the X-ray measurements at the ID9 beam line of the ESRF synchrotron (Grenoble) and N. R. Serebryanaya for valuable discussions. I.T. and A.G. appreciate support of DFG grant 436 RUS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Eremets, M., Gavriliuk, A., Trojan, I. et al. Single-bonded cubic form of nitrogen. Nature Mater 3, 558–563 (2004). https://doi.org/10.1038/nmat1146
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat1146
This article is cited by
-
Materials under extreme conditions using large X-ray facilities
Nature Reviews Methods Primers (2023)
-
Universal diamond edge Raman scale to 0.5 terapascal and implications for the metallization of hydrogen
Nature Communications (2023)
-
Benzene-like N6 hexazine rings
Nature Chemistry (2023)
-
Structure determination of ζ-N2 from single-crystal X-ray diffraction and theoretical suggestion for the formation of amorphous nitrogen
Nature Communications (2023)
-
Aromatic hexazine [N6]4− anion featured in the complex structure of the high-pressure potassium nitrogen compound K9N56
Nature Chemistry (2023)