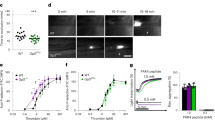
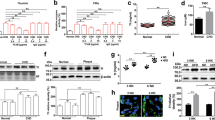
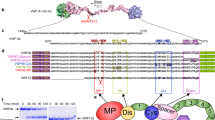
Abstract
Tissue factor (TF) is an essential enzyme activator that forms a catalytic complex with FVIIa and initiates coagulation by activating FIX and FX, ultimately resulting in thrombin formation1. TF is found in adventitia of blood vessels2 and the lipid core of atherosclerotic plaques3. In unstable coronary syndromes, plaque rupture initiates coagulation by exposing TF to blood4,5. Biologically active TF has been detected in vessel walls and circulating blood6. Elevated intravascular TF has been reported in diverse pro-thrombotic syndromes such as myocardial infarction, sepsis, anti-phospholipid syndrome and sickle-cell disease7,8,9,10. It is unclear how TF circulates, although it may be present in pro-coagulant microparticles11,12. We now report identification of a form of human TF generated by alternative splicing. Our studies indicate that alternatively spliced human tissue factor (asHTF) contains most of the extracellular domain of TF but lacks a transmembrane domain and terminates with a unique peptide sequence. asHTF is soluble, circulates in blood, exhibits pro-coagulant activity when exposed to phospholipids, and is incorporated into thrombi. We propose that binding of asHTF to the edge of thrombi contributes to thrombus growth by creating a surface that both initiates and propagates coagulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Bach, R.R. Initiation of coagulation by tissue factor. CRC Crit. Rev. Biochem. 23, 339–368 (1988).
Nemerson, Y. & Gentry, R. An ordered addition, essential activation model of the tissue factor pathway of coagulation: evidence for a conformational cage. Biochemistry 25, 4020–4033 (1986).
Zaman, A.G., Helft, G., Worthley, S.G. & Badimon, J.J. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis 149, 251–266 (2000).
Zeldis, S.M., Nemerson, Y., Pitlick, F.A. & Lentz, T.L. Tissue factor (thromboplastin): localization to plasma membranes by peroxidase-conjugated antibodies. Science 175, 766–768 (1972).
Baumgartner, H.R., Turitto, V.T. & Weiss, H.J. Effects of shear rate on platelet interaction with subendothelium in citrated and native blood. II. Relationships among platelet adhesion, thrombus dimensions, and fibrin formation. J. Lab. Clin. Med. 95, 208–221 (1980).
Giesen, P.L. et al. Blood-borne tissue factor: another view of thrombosis. Proc. Natl. Acad. Sci. USA 96, 2311–2315 (1999).
Suefuji, H. et al. Increased plasma tissue factor levels in acute myocardial infarction. Am. Heart J. 134, 253–259 (1997).
Gando, S., Nanzaki, S., Sasaki, S., Aoi, K. & Kemmotsu, O. Activation of the extrinsic coagulation pathway in patients with severe sepsis and septic shock. Crit. Care Med. 26, 2005–2009 (1998).
Amengual, O., Atsumi, T., Khamashta, M.A. & Hughes, G.R. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb. Haemost. 79, 276–281 (1998).
Key, N.S. et al. Whole blood tissue factor procoagulant activity is elevated in patients with sickle cell disease. Blood 91, 4216–4223 (1998).
Mallat, Z. et al. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 99, 348–353 (1999).
Mallat, Z. et al. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 101, 841–843 (2000).
Kelley, R.F., Costas, K.E., O'Connell, M.P. & Lazarus, R.A. Analysis of the factor VIIa binding site on human tissue factor: effects of tissue factor mutations on the kinetics and thermodynamics of binding. Biochemistry 34, 10383–10392 (1995).
Rosseau, S. et al. Monocyte migration through the alveolar epithelial barrier: adhesion molecule mechanisms and impact of chemokines. J. Immunol. 164, 427–435 (2000).
Carson, S. & Yoder, S. Monoclonal antibodies against the C-terminal peptide of human tissue factor for studies of the cytoplasmic domain. Blood Coagul. Fibrinolysis 3, 779–787 (1992).
Badimon, L., Badimon, J.J., Galvez, A., Chesebro, J.H. & Fuster, V. Influence of arterial damage and wall shear rate on platelet deposition. Ex vivo study in a swine model. Arteriosclerosis 6, 312–320 (1986).
Guo, W. et al. Effect of all-trans retinoic acid and arsenic trioxide on tissue factor expression in acute promyelocytic leukemia cells. Chin. Med. J. (Engl.) 114, 30–34 (2001).
Berckmans, R.J. et al. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb. Haemost. 85, 639–646 (2001).
Parry, G.C., Erlich, J.H., Carmeliet, P., Luther, T. & Mackman, N. Low levels of tissue factor are compatible with development and hemostasis in mice. J. Clin. Invest. 101, 560–569 (1998).
Pawlinski, R. et al. Tissue factor deficiency causes cardiac fibrosis and left ventricular dysfunction. Proc. Natl. Acad. Sci. USA 99, 15333–15338 (2002).
Berg, H.C. Diffusion to capture. In Random Walks in Biology Chap. 3, 37–47 (Princeton University Press, Princeton, New Jersey, 1993).
Ambrose, J.A., Almeida, O.D., Sharma, S.K., Dangas, G. & Ratner, D.E. Angiographic evolution of intracoronary thrombus and dissection following percutaneous transluminal coronary angioplasty (the Thrombolysis and Angioplasty in Unstable Angina [TAUSA] trial). Am. J. Cardiol. 79, 559–563 (1997).
Turitto, V.T. & Hall, C.L. Mechanical factors affecting hemostasis and thrombosis. Thromb. Res. 92, S25–S31 (1998).
Thiruvikraman, S.V. et al. In situ localization of tissue factor in human atherosclerotic plaques by binding of digoxigenin-labeled factors VIIa and X. Lab. Invest. 75, 451–461 (1996).
Grabowski, E.F. Platelet aggregation in flowing blood at a site of injury to an endothelial cell monolayer: quantitation and real-time imaging with the TAB monoclonal antibody. Blood 75, 390–398 (1990).
Acknowledgements
We thank M.B. Taubman, M.C. Moschella, and J.T. Fallon for discussions and advice, S. Carson for monoclonal antibody against membrane-bound TF, E. Grabowski for parallel-plate chamber, J.J. Badimon for specimens of ex vivo thrombi, and H. Vaananen for technical assistance. V.Y.B., V.B., J.H., O.V., M.L. and Y.N. are partially supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bogdanov, V., Balasubramanian, V., Hathcock, J. et al. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med 9, 458–462 (2003). https://doi.org/10.1038/nm841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm841
This article is cited by
-
Potential utility of a multi-component coagulation factor panel to calculate MELD scores and assess the risk of portal vein thrombosis in chronic liver disease
BMC Gastroenterology (2023)
-
Uncovering emergent phenotypes in endothelial cells by clustering of surrogates of cardiovascular risk factors
Scientific Reports (2022)
-
Expression of tissue factor mRNA in thrombosis associated with antiphospholipid syndrome
Journal of Thrombosis and Thrombolysis (2021)
-
Alternative splicing in endothelial cells: novel therapeutic opportunities in cancer angiogenesis
Journal of Experimental & Clinical Cancer Research (2020)
-
Beyond thrombosis: the impact of tissue factor signaling in cancer
Journal of Hematology & Oncology (2020)