Abstract
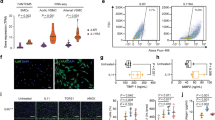
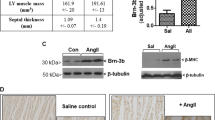
We recently isolated a Krüppel-like zinc-finger transcription factor 5 (KLF5; also known as BTEB2 and IKLF), which is markedly induced in activated vascular smooth-muscle cells and fibroblasts. Here we describe our analysis of the in vivo function of KLF5 using heterozygous KLF5-knockout mice (Klf5+/−). In response to external stress, Klf5+/− mice showed diminished levels of arterial-wall thickening, angiogenesis, cardiac hypertrophy and interstitial fibrosis. Also, angiotensin II induced expression of KLF5, which in turn activated platelet-derived growth factor-A (PDGF-A) and transforming growth factor-β (TGF-β) expression. In addition, we determined that KLF5 interacted with the retinoic-acid receptor (RAR), that synthetic RAR ligands modulated KLF5 transcriptional activity, and that in vivo administration of RAR ligands affected stress responses in the cardiovascular system in a KLF5-dependent manner. KLF5 thus seems to be a key element linking external stress and cardiovascular remodeling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Carmeliet, P. & Collen, D. Transgenic mouse models in angiogenesis and cardiovascular disease. J. Pathol. 190, 387–405 (2000).
Libby, P. Changing concepts of atherogenesis. J. Intern. Med. 247, 349–358 (2000).
Kuro-o, M. et al. Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J. Biol. Chem. 264, 18272–82725 (1989).
Manabe, I. et al. Isolation of the embryonic form of smooth muscle myosin heavy chain (SMemb/NMHC-B) gene and characterization of its 5′-flanking region. Biochem. Biophys. Res. Commun. 239, 598–605 (1997).
Aikawa, M. et al. Phenotypic modulation of smooth-muscle cells during progression of human atherosclerosis as determined by altered expression of myosin heavy chain isoforms. Ann. NY Acad. Sci. 748, 578–585 (1995).
Watanabe, N. et al. BTEB2, a Kruppel-like transcription factor, regulates expression of the SMemb/nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ. Res. 85, 182–191 (1999).
Bieker, J.J. Kruppel-like factors: Three fingers in many pies. J. Biol. Chem. 276, 34355–34358 (2001).
Dang, D.T., Pevsner, J. & Yang, V.W. The biology of the mammalian Kruppel-like family of transcription factors. Int. J. Biochem. Cell Biol. 32, 1103–1121 (2000).
Black, A.R., Black, J.D. & Azizkhan-Clifford, J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J. Cell Physiol. 188, 143–160 (2001).
Hoshino, Y. et al. Regulated expression of the BTEB2 transcription factor in vascular smooth-muscle cells: Analysis of developmental and pathological expression profiles shows implications as a predictive factor for restenosis. Circulation 102, 2528–2534 (2000).
Kawai-Kowase, K., Kurabayashi, M., Hoshino, Y., Ohyama, Y. & Nagai, R. Transcriptional activation of the zinc finger transcription factor BTEB2 gene by Egr-1 through mitogen-activated protein kinase pathways in vascular smooth-muscle cells. Circ. Res. 85, 787–795 (1999).
Berk, B.C. Vascular smooth muscle growth: Autocrine growth mechanisms. Physiol. Rev. 81, 999–1030 (2001).
Matsusaka, T., Katori, H., Inagami, T., Fogo, A. & Ichikawa, I. Communication between myocytes and fibroblasts in cardiac remodeling in angiotensin chimeric mice. J. Clin. Invest. 103, 1451–1458 (1999).
Weir, M.R. & Dzau, V.J. The renin-angiotensin-aldosterone system: A specific target for hypertension management. Am. J. Hypertens. 12, 205S–213S (1999).
Powell, D.W. et al. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. 277, C1–9 (1999).
Karlsson, L., Lindahl, P., Heath, J.K. & Betsholtz, C. Abnormal gastrointestinal development in PDGF-A and PDGFR-α deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development 127, 3457–66 (2000).
Zdunek, M., Silbiger, S., Lei, J. & Neugarten, J. Protein kinase CK2 mediates TGF-β1-stimulated type IV collagen gene transcription and its reversal by estradiol. Kidney Int. 60, 2097–2108 (2001).
Umemiya, H. et al. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J. Med. Chem. 40, 4222–34 (1997).
Eyrolles, L. et al. Retinobenzoic acids. 6. Retinoid antagonists with a heterocyclic ring. J. Med. Chem. 37, 1508–17 (1994).
Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 275, 2247–50 (2000).
Silverman, E.S. & Collins, T. Pathways of Egr-1-mediated gene transcription in vascular biology. Am. J. Pathol. 154, 665–70 (1999).
Valen, G., Yan, Z.Q. & Hansson, G.K. Nuclear factor κ-B and the heart. J. Am. Coll. Cardiol. 38, 307–14 (2001).
Yamauchi-Takihara, K. & Kishimoto, T. A novel role for STAT3 in cardiac remodeling. Trends Cardiovasc. Med. 10, 298–303 (2000).
Glass, C.K. & Rosenfeld, M.G. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14, 121–41 (2000).
Shindo, T. et al. ADAMTS-1: A metalloproteinase-disintegrin essential for normal growth, fertility, and organ morphology and function. J. Clin. Invest. 105, 1345–52 (2000).
Shindo, T. et al. Vascular abnormalities and elevated blood pressure in mice lacking adrenomedullin gene. Circulation 104, 1964–71 (2001).
Oh-hashi, Y. et al. Elevated sympathetic nervous activity in mice deficient in αCGRP. Circ. Res. 89, 983–90 (2001).
Moroi, M. et al. Interaction of genetic deficiency of endothelial nitric oxide, gender, and pregnancy in vascular response to injury in mice. J. Clin. Invest. 101, 1225–32 (1998).
Akishita, M. et al. Estrogen inhibits cuff-induced intimal thickening of rat femoral artery: Effects on migration and proliferation of vascular smooth-muscle cells. Atherosclerosis 130, 1–10 (1997).
Wang, H.D. et al. Role of NADPH oxidase in the vascular hypertrophic and oxidative stress response to angiotensin II in mice. Circ. Res. 88, 947–53 (2001).
Komuro, I. et al. Stretching cardiac myocytes stimulates protooncogene expression. J. Biol. Chem. 265, 3595–8 (1990).
Kihm, A.J., Hershey, J.C., Haystead, T.A., Madsen, C.S. & Owens, G.K. Phosphorylation of the rRNA transcription factor upstream binding factor promotes its association with TATA binding protein. Proc. Natl. Acad. Sci. USA 95, 14816–20 (1998).
Manabe, I. & Owens, G.K. Recruitment of serum response factor and hyperacetylation of histones at smooth muscle-specific regulatory regions during differentiation of a novel P19-derived in vitro smooth muscle differentiation system. Circ. Res. 88, 1127–1134 (2001).
Acknowledgements
We thank T. Collins for the PDGF-A reporter constructs, CT. Teng for the human IKLF construct; SF. Yet for the GKLF construct; R. Urrutia for the TIEG construct; and H. Kumagaya for the S180 cell line. This study was supported in part by a Grant-in-Aid for Millennium Projects from the Ministry of Health, Labor and Welfare, Japan and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Shindo, T., Manabe, I., Fukushima, Y. et al. Krüppel-like zinc-finger transcription factor KLF5/BTEB2 is a target for angiotensin II signaling and an essential regulator of cardiovascular remodeling. Nat Med 8, 856–863 (2002). https://doi.org/10.1038/nm738
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm738
This article is cited by
-
The KLF7/PFKL/ACADL axis modulates cardiac metabolic remodelling during cardiac hypertrophy in male mice
Nature Communications (2023)
-
Transient regulation of RNA methylation in human hematopoietic stem cells promotes their homing and engraftment
Leukemia (2023)
-
Zinc finger and BTB domain-containing protein 20 aggravates angiotensin II-induced cardiac remodeling via the EGFR-AKT pathway
Journal of Molecular Medicine (2022)
-
Exosomes in cardiovascular diseases: a blessing or a sin for the mankind
Molecular and Cellular Biochemistry (2022)
-
FIH-1-modulated HIF-1α C-TAD promotes acute kidney injury to chronic kidney disease progression via regulating KLF5 signaling
Acta Pharmacologica Sinica (2021)