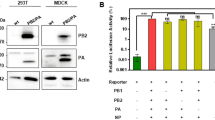
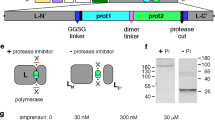
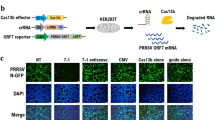
Abstract
RNA interference silences gene expression through short interfering 21–23-mer double-strand RNA segments that guide mRNA degradation in a sequence-specific fashion. Here we report that siRNAs inhibit virus production by targeting the mRNAs for either the HIV-1 cellular receptor CD4, the viral structural Gag protein or green fluorescence protein substituted for the Nef regulatory protein. siRNAs effectively inhibit pre- and/or post-integration infection events in the HIV-1 life cycle. Thus, siRNAs may have potential for therapeutic intervention in HIV-1 and other viral infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Sharp, P.A. RNA interference-2001. Genes Dev. 15, 485–490 (2001).
Zamore, P.D. RNA interference: listening to the sound of silence. Nature Struct. Biol. 8, 746–750 (2001).
Bernstein, E., Caudy, A.A., Hammond, S.M. & Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409, 363–366 (2001).
Ketting, R.F., Haverkamp, T.H., van Luenen, H.G. & Plasterk, R.H. Mut-7 of C. elegans, required for transposon silencing and RNA interference, is a homolog of Werner syndrome helicase and RnaseD. Cell 99, 133–141 (1999).
Tabara, H. et al. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell 99, 123–132 (1999).
Jones, A.R. & Schedl, T. Mutations in gld-1, a female germ cell-specific tumor suppressor gene in Caenorhabditis elegans, affect a conserved domain also found in Src-associated protein Sam68. Genes Dev. 9, 1491–1504 (1995).
Gaudet, J., Van der Elst, I. & Spence, A.M. Post-transcriptional regulation of sex determination in Caenorhabditis elegans: widespread expression of the sex-determining gene fem-1 in both sexes. Mol. Biol. Cell 7, 1107–1121 (1996).
Pal-Bhadra, M., Bhadra, U. & Birchler, J.A. Cosuppression of nonhomologous transgenes in Drosophila involves mutually related endogenous sequences. Cell 99, 35–46 (1999).
Ratcliff, F., Harrison, B.D. & Baulcombe, D.C. A similarity between viral defense and gene silencing in plants. Science 276, 1558–1560 (1997).
Waterhouse, P.M., Wang, M.-B. & Lough, T. Gene silencing as an adaptive defence against viruses. Nature 411, 834–842 (2001).
Kumar, M. & Carmichael, G.G. Antisense RNA: function and fate of duplex RNA in cells of higher eukaryotes. Microbiol. Mol. Biol. Rev. 62, 1415–1434 (1998).
Stark, G.R., Kerr, I.M., Williams, B.R., Silverman, R.H. & Schreiber, R.D. How cells respond to interferons. Annu. Rev. Biochem. 67, 227–264 (1998).
Elbashir, S.M., Lendeckel, W. & Tuschl, T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 15, 188–200 (2001).
Elbashir, S.M., Martinez, J., Patkaniowska, A., Lendeckel, W. & Tuschl, T. Functional anatomy of siRNAs for mediating efficient RNAi in Drosophila melanogaster embryo lysate. EMBO J. 20, 6877–6888 (2001).
Klatzmann, D. et al. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312, 767–768 (1984).
Maddon, P.J. et al. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell 47, 333–348 (1986).
Chackerian, B., Long, E.M., Luciw, P.A. & Overbaugh, J. HIV-1 co-receptors participates in post-entry stages of the virus replication cycle and function in SIV infection. J. Virol. 71, 3932–3939 (1997).
Pavlakis, G.N., Schwartz, S., D'Agostino, D.M. & Felber, B.K. Structure, splicing, and regulation of expression of HIV-1: a model for the general organization of lentiviruses and other complex retroviruses. in Annual Review of AIDS Research. (eds. Kennedy, R., Wong-Staal, F. & Koff, W.C.) 41–63 (Marcel Dekker, New York, 1991).
Kim, S., Byrn, R., Groopman, J. & Baltimore, D. Temporal aspects of DNA and RNA synthesis during human immunodeficiency virus infection: evidence for differential gene expression. J. Virol. 63, 3708–3713 (1989).
Wu, Y. & Marsh, J.W. Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293, 1503–1506 (2001).
Page, K.A., Liegler, T. & Feinberg, M.B. Use of a green fluorescent protein as a marker for human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retroviruses 13, 1077–1081 (1997).
Samson, M. et al. Resistance to HIV-1 infection in caucasian individualos bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996).
Liu, R. et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996).
Bitko, V. & Barik, S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 34, 1–11 (2001).
Hammond, S.M., Bernstein, E., Beach, D. & Hannon, G.J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404, 293–296 (2000).
Hammond, S.M., Boettcher, S., Caudy, A.A., Kobayashi, R. & Hannon, G.J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293, 1146–1150 (2001).
Zapp, M.L. & Green, M.R. Sequence-specific RNA binding by the HIV-1 Rev protein. Nature 342, 714–716 (1989).
Malim, M.H. et al. HIV-1 structural gene expression requires binding of the Rev trans- activator to its RNA target sequence. Cell 60, 675–683 (1990).
Cochrane, A.W., Chen, C.H. & Rosen, C.A. Specific interaction of the human immunodeficiency virus Rev protein with a structured region in the env mRNA. Proc. Natl. Acad. Sci. USA 87, 1198–1202 (1990).
Brummelkamp, T.R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002).
Paddison, P.J., Caudy, A.A., Bernstein, E., Hannon, G.J. & Conklin, D.S. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 16, 948–958 (2002).
Sui, G. et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 5515–5520 (2002).
Yu, J.-Y., DeRuiter, S.L. & Turner, D.L. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc. Natl. Acad. Sci. USA 99, 6047–6052 (2002).
Paul, C.P., Good, P.D., Winer, I. & Engelke, D.R. Effective expression of small interfering RNA in human cells. Nature Biotech. 20, 505–508 (2002).
McManus, M.T., Petersen, C.P., Haines, B.B., Chen, J. & Sharp, P.A. Gene silencing using micro-RNA designed hairpins. RNA 8, 1–9 (2002).
Grishok, A. et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 106, 23–34 (2001).
Hutvagner, G. et al. A cellular function for the RNA-interference enzyme DICER in the maturation of the let-7 small temporal RNA. Science 293, 834–838 (2001).
Ketting, R.F. et al. DICER function in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 15, 2654–2659 (2001).
Rougvie, A.E. Control of developmental timing in animals. Nature Rev. Genet. 2, 690–701 (2001).
Banerjee, D. & Slack, F. Control of developmental timing by small temporal RNAs: a paradigm for RNA-mediated regulation of gene expression. Bioessays 24, 119–129 (2002).
Dorman, N. & Lever, A.M. RNA-based gene therapy for HIV infection. HIV Med 2, 114–122 (2001).
James, H.A. & Gibson, I. The therapeutic potential of ribozymes. Blood 91, 371–382 (1998).
Lee, N.S. et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nature Biotech. 20, 500–505 (2002).
Shankar, P., Xu, Z. & Lieberman, J. Viral-specific cytotoxic T lymphocytes lyse human immunodeficiency virus-infected primary T lymphocytes by the granule exocytosis pathway. Blood 94, 3084–3093 (1999).
Ratner, L. et al. Complete nucleotide sequences of functional clones of the AIDS virus. AIDS Res. Hum. Retroviruses 3, 57–69 (1987).
Acknowledgements
We thank V. François-Bongarçon for help and technical assistance; H. Cargill for help with Fig. 6; and J. Doench for helpful discussions and comments. The following reagents were obtained through the AIDS Research and Reference Reagent Program Division of AIDS, NIAID, NIH: HeLaT4+ and T4pMV7 were provided by R. Axel, pR7-GFP was provided by K. Page and M. Feinberg and Magi-CCR5 was provided by J. Overbaugh. This work was supported by grants NIH F32 AI10523 (to C.D.N.), NIH T32-GM07748 (to M.F.M.), NIH AI 35502 (to R.G.C.), NIH AI 45306 (to P.S.) and NIH MERIT grant R37-GM34277, NCI grant PO1-CA42063 and partially by NCI Cancer Center Support (Core) grant P30-CA14051 (to P.A.S.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors of this paper have applied for a patent that will be owned by the Massachusetts Institute of Technology. It is possible that in the future the authors will receive royalties from this patent.
Supplementary information
Rights and permissions
About this article
Cite this article
Novina, C., Murray, M., Dykxhoorn, D. et al. siRNA-directed inhibition of HIV-1 infection. Nat Med 8, 681–686 (2002). https://doi.org/10.1038/nm725
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm725
This article is cited by
-
Editing out HIV: application of gene editing technology to achieve functional cure
Retrovirology (2021)
-
shRNA transgenic swine display resistance to infection with the foot-and-mouth disease virus
Scientific Reports (2021)
-
SiRNA silencing efficacy prediction based on a deep architecture
BMC Genomics (2018)
-
Intranasal drug delivery of small interfering RNA targeting Beclin1 encapsulated with polyethylenimine (PEI) in mouse brain to achieve HIV attenuation
Scientific Reports (2017)
-
Electro-Magnetic Nano-Particle Bound Beclin1 siRNA Crosses the Blood–Brain Barrier to Attenuate the Inflammatory Effects of HIV-1 Infection in Vitro
Journal of Neuroimmune Pharmacology (2017)