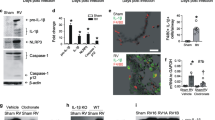
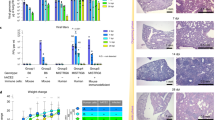
Abstract
Rhinoviruses cause serious morbidity and mortality as the major etiological agents of asthma exacerbations and the common cold. A major obstacle to understanding disease pathogenesis and to the development of effective therapies has been the lack of a small-animal model for rhinovirus infection. Of the 100 known rhinovirus serotypes, 90% (the major group) use human intercellular adhesion molecule-1 (ICAM-1) as their cellular receptor and do not bind mouse ICAM-1; the remaining 10% (the minor group) use a member of the low-density lipoprotein receptor family and can bind the mouse counterpart. Here we describe three novel mouse models of rhinovirus infection: minor-group rhinovirus infection of BALB/c mice, major-group rhinovirus infection of transgenic BALB/c mice expressing a mouse-human ICAM-1 chimera and rhinovirus-induced exacerbation of allergic airway inflammation. These models have features similar to those observed in rhinovirus infection in humans, including augmentation of allergic airway inflammation, and will be useful in the development of future therapies for colds and asthma exacerbations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lemanske, R.F., Jr. et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J. Allergy Clin. Immunol. 116, 571–577 (2005).
Kaiser, L. et al. Chronic rhinoviral infection in lung transplant recipients. Am. J. Respir. Crit. Care Med. 174, 1392–1399 (2006).
Johnston, S.L. et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. Br. Med. J. 310, 1225–1229 (1995).
Corne, J.M. et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 359, 831–834 (2002).
Chauhan, A.J. et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus-induced asthma in children. Lancet 361, 1939–1944 (2003).
Grissell, T.V. et al. Interleukin-10 gene expression in acute virus-induced asthma. Am. J. Respir. Crit. Care Med. 172, 433–439 (2005).
Papi, A. et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am. J. Respir. Crit. Care Med. 173, 1114–1121 (2006).
Bertino, J.S. Cost burden of viral respiratory infections: issues for formulary decision makers. Am. J. Med. 112 (Suppl. 6A), 42S–49S (2002).
Lomax, N.B. & Yin, F.H. Evidence for the role of the P2 protein of human rhinovirus in its host range change. J. Virol. 63, 2396–2399 (1989).
Tuthill, T.J. et al. Mouse respiratory epithelial cells support efficient replication of human rhinovirus. J. Gen. Virol. 84, 2829–2836 (2003).
Greve, J.M. et al. The major human rhinovirus receptor is ICAM-1. Cell 56, 839–847 (1989).
Register, R.B., Uncapher, C.R., Naylor, A.M., Lineberger, D.W. & Colonno, R.J. Human-murine chimeras of ICAM-1 identify amino acid residues critical for rhinovirus and antibody binding. J. Virol. 65, 6589–6596 (1991).
Staunton, D.E., Gaur, A., Chan, P.Y. & Springer, T.A. Internalization of a major group human rhinovirus does not require cytoplasmic or transmembrane domains of ICAM-1. J. Immunol. 148, 3271–3274 (1992).
Liao, F. et al. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3α. J. Immunol. 162, 186–194 (1999).
Nakayama, T. et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 alpha/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int. Immunol. 13, 95–103 (2001).
Fraenkel, D.J. et al. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am. J. Respir. Crit. Care Med. 151, 879–886 (1995).
Grunberg, K. et al. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am. J. Respir. Crit. Care Med. 156, 609–616 (1997).
Inoue, D. et al. Mechanisms of mucin production by rhinovirus infection in cultured human airway epithelial cells. Respir. Physiol. Neurobiol. 154, 484–499 (2006).
Wark, P.A. et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201, 937–947 (2005).
Contoli, M. et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat. Med. 12, 1023–1026 (2006).
Ank, N. et al. Lambda interferon (IFN-λ), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J. Virol. 80, 4501–4509 (2006).
Bartlett, N.W., Buttigieg, K., Kotenko, S.V. & Smith, G.L. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J. Gen. Virol. 86, 1589–1596 (2005).
Harris, J.R. & Racaniello, V.R. Changes in rhinovirus protein 2C allow efficient replication in mouse cells. J. Virol. 77, 4773–4780 (2003).
Harris, J.R. & Racaniello, V.R. Amino acid changes in proteins 2B and 3A mediate rhinovirus type 39 growth in mouse cells. J. Virol. 79, 5363–5373 (2005).
Wark, P.A. & Gibson, P.G. Asthma exacerbations. 3: Pathogenesis. Thorax 61, 909–915 (2006).
Groneberg, D.A. et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology 40, 367–373 (2002).
Murray, C.S. et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax 61, 376–382 (2006).
Green, R.M. et al. Synergism between allergens and viruses and risk of hospital admission with asthma: case-control study. Br. Med. J. 324, 763 (2002).
Hewson, C.A., Jardine, A., Edwards, M.R., Laza-Stanca, V. & Johnston, S.L. Toll-like receptor 3 is induced by and mediates antiviral activity against rhinovirus infection of human bronchial epithelial cells. J. Virol. 79, 12273–12279 (2005).
Murphy, B.R., Prince, G.A., Lawrence, L.A., Croen, K.D. & Collins, P.L. Detection of respiratory syncytial virus (RSV) infected cells by in situ hybridization in the lungs of cotton rats immunized with formalin-inactivated virus or purified RSV F and G glycoprotein subunit vaccine and challenged with RSV. Virus Res. 16, 153–162 (1990).
Acknowledgements
This work was supported by Medical Research Council UK grant number G9824522, GlaxoSmithKline, Sanofi Pasteur and Asthma UK grant numbers 03/073, 04/052, 05/067 and 06/050.
Author information
Authors and Affiliations
Contributions
S.L.J. conceived the studies and was principle investigator; all authors contributed to the design and execution of the experiments, helped draft the manuscript and approved the final version for publication.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–3 and Supplementary Methods (PDF 755 kb)
Rights and permissions
About this article
Cite this article
Bartlett, N., Walton, R., Edwards, M. et al. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med 14, 199–204 (2008). https://doi.org/10.1038/nm1713
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1713
This article is cited by
-
Rhinovirus infection induces secretion of endothelin-1 from airway epithelial cells in both in vitro and in vivo models
Respiratory Research (2023)
-
IL-25 blockade augments antiviral immunity during respiratory virus infection
Communications Biology (2022)
-
Different Phenotypes in Asthma: Clinical Findings and Experimental Animal Models
Clinical Reviews in Allergy & Immunology (2022)
-
Human rhinovirus serotypes induces different immune responses
Virology Journal (2021)
-
Direct effects of mast cell proteases, tryptase and chymase, on bronchial epithelial integrity proteins and anti-viral responses
BMC Immunology (2021)