Abstract
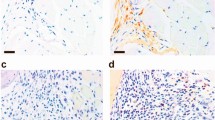
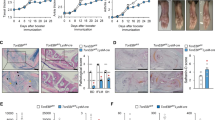
The dendritic cell immunoreceptor (official gene symbol Clec4a2, called Dcir here) is a C-type lectin receptor expressed mainly in dendritic cells (DCs) that has a carbohydrate recognition domain in its extracellular portion and an immunoreceptor tyrosine–based inhibitory motif, which transduces negative signals into cells, in its cytoplasmic portion1. We found high Dcir expression in the joints of two mouse rheumatoid arthritis models2,3,4. Because the structural characteristics of Dcir suggest that it may have an immune regulatory role, and because autoimmune-related genes are mapped to the DCIR locus in humans, we generated Dcir−/− mice to learn more about the pathological roles of this molecule. We found that aged Dcir−/− mice spontaneously develop sialadenitis and enthesitis associated with elevated serum autoantibodies. Dcir−/− mice showed a markedly exacerbated response to collagen-induced arthritis. The DC population was expanded excessively in aged and type II collagen–immunized Dcir−/− mice. Upon treatment with granulocyte-macrophage colony–stimulating factor, Dcir−/− mouse–derived bone marrow cells (BMCs) differentiated into DCs more efficiently than did wild-type BMCs, owing to enhanced signal transducer and activator of transcription-5 phosphorylation. These observations indicate that Dcir is a negative regulator of DC expansion and has a crucial role in maintaining the homeostasis of the immune system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bates, E.E. et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 163, 1973–1983 (1999).
Iwakura, Y. et al. Induction of inflammatory arthropathy resembling rheumatoid arthritis in mice transgenic for HTLV-I. Science 253, 1026–1028 (1991).
Horai, R. et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist–deficient mice. J. Exp. Med. 191, 313–320 (2000).
Fujikado, N., Saijo, S. & Iwakura, Y. Identification of arthritis-related gene clusters by microarray analysis of two independent mouse models for rheumatoid arthritis. Arthritis Res. Ther. 8, R100 1–13 (2006).
Franceschini, F. & Cavazzana, I. Anti-Ro/SSA and La/SSB antibodies. Autoimmunity 38, 55–63 (2005).
Shparago, N. et al. IL-10 selectively regulates murine Ig isotype switching. Int. Immunol. 8, 781–790 (1996).
Nakae, S. et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 100, 5986–5990 (2003).
Nakae, S., Nambu, A., Sudo, K. & Iwakura, Y. Suppression of immune induction of collagen-induced arthritis in IL-17–deficient mice. J. Immunol. 171, 6173–6177 (2003).
Helenius, L.M. et al. Focal sialadenitis in patients with ankylosing spondylitis and spondyloarthropathy: a comparison with patients with rheumatoid arthritis or mixed connective tissue disease. Ann. Rheum. Dis. 60, 744–749 (2001).
Huang, X. et al. Cloning and characterization of a novel ITIM containing lectin-like immunoreceptor LLIR and its two transmembrane region deletion variants. Biochem. Biophys. Res. Commun. 281, 131–140 (2001).
Richard, M. et al. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol. Immunol. 43, 1716–1721 (2006).
Daigle, I. et al. Death receptors bind SHP-1 and block cytokine-induced anti-apoptotic signaling in neutrophils. Nat. Med. 8, 61–67 (2002).
Kanazawa, N. et al. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J. Invest. Dermatol. 118, 261–266 (2002).
Wo Tsui, H., Siminovitch, K.A., de Souza, L. & Tsui, F.W. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat. Genet. 4, 124–129 (1993).
Shultz, L.D. et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell 73, 1445–1454 (1993).
Chen, M. et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 311, 1160–1164 (2006).
Stranges, P.B. et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity 26, 629–641 (2007).
Wandstrat, A. & Wakeland, E. The genetics of complex autoimmune diseases: non-MHC susceptibility genes. Nat. Immunol. 2, 802–809 (2001).
Lorentzen, J.C. et al. Association of arthritis with a gene complex encoding C-type lectin–like receptors. Arthritis Rheum. 56, 2620–2632 (2007).
Saijo, S. et al. Suppression of autoimmune arthritis in interleukin-1–deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 46, 533–544 (2002).
Zhao, P., Liu, W. & Cui, Y. Rapid immune reconstitution and dendritic cell engraftment post–bone marrow transplantation with heterogeneous progenitors and GM-CSF treatment. Exp. Hematol. 34, 951–964 (2006).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Yonezawa, T., Katoh, K. & Obara, Y. Existence of GPR40 functioning in a human breast cancer cell line, MCF-7. Biochem. Biophys. Res. Commun. 314, 805–809 (2004).
Acknowledgements
We thank all of the members of the laboratory for their excellent animal care. This research was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Ministry of Health and Welfare of Japan. N.F. was supported by a fellowship from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
N.F. mainly contributed throughout this work in collaboration with S. Saijo. T.Y. did quantitative real-time RT-PCR experiments. K. Shimamori, A.I. and S. Sugai provided technical support. H.K. and K. Sudo did embryonic stem cell culture and produced chimeric mice. M.N. carried out histological analysis. Y.I. organized and supervised the project and edited the draft manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–7 and Supplementary Methods (PDF 3156 kb)
Rights and permissions
About this article
Cite this article
Fujikado, N., Saijo, S., Yonezawa, T. et al. Dcir deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med 14, 176–180 (2008). https://doi.org/10.1038/nm1697
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1697
This article is cited by
-
C-type lectin receptor CLEC4A2 promotes tissue adaptation of macrophages and protects against atherosclerosis
Nature Communications (2022)
-
C-type lectin receptor DCIR contributes to hippocampal injury in acute neurotropic virus infection
Scientific Reports (2021)
-
TARM1 contributes to development of arthritis by activating dendritic cells through recognition of collagens
Nature Communications (2021)
-
Identification of immune-related genes with prognostic significance in the microenvironment of cutaneous melanoma
Virchows Archiv (2021)
-
C-type lectins in immunity and homeostasis
Nature Reviews Immunology (2018)