Abstract
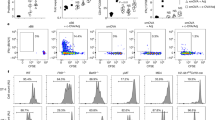
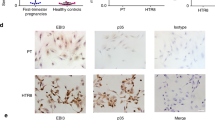
A successful pregnancy requires synchronized adaptation of maternal immune-endocrine mechanisms to the fetus. Here we show that galectin-1 (Gal-1), an immunoregulatory glycan-binding protein, has a pivotal role in conferring fetomaternal tolerance. Consistently with a marked decrease in Gal-1 expression during failing pregnancies, Gal-1–deficient (Lgals1−/−) mice showed higher rates of fetal loss compared to wild-type mice in allogeneic matings, whereas fetal survival was unaffected in syngeneic matings. Treatment with recombinant Gal-1 prevented fetal loss and restored tolerance through multiple mechanisms, including the induction of tolerogenic dendritic cells, which in turn promoted the expansion of interleukin-10 (IL-10)–secreting regulatory T cells in vivo. Accordingly, Gal-1's protective effects were abrogated in mice depleted of regulatory T cells or deficient in IL-10. In addition, we provide evidence for synergy between Gal-1 and progesterone in the maintenance of pregnancy. Thus, Gal-1 is a pivotal regulator of fetomaternal tolerance that has potential therapeutic implications in threatened pregnancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
07 May 2009
In the version of this article initially published, the plot labeled “Stress + Gal-1” duplicated the plot labeled “Control” for the IL-12p70 staining in Figure 2f. The corrected plots have now been provided in the HTML and PDF versions of the article. Two sentences were omitted from the section on purification of uterine DCs in the Methods. The sentences should have read: “We obtained purified DCs from uterine tissue in very low numbers. Thus, we pooled isolated cells from each group and used this cell cocktail for the isotype control staining.” The error has been corrected in the HTML and PDF versions of the article. In Figure 4f, the lanes of the western blot were merged inappropriately. The properly presented blot, in which the gel lanes have been separated to indicate that the samples were not originally run side by side, has been provided in the PDF and HTML versions of the article. The standard curve of the cytometric bead array kit used to generate the cytokine data can be found in the revised supplementary information available online.
References
Trowsdale, J. & Betz, A.G. Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 (2006).
Chaouat, G., Ledee-Bataille, N., Chea, K.B. & Dubanchet, S. Cytokines and implantation. Chem. Immunol. Allergy 88, 34–63 (2005).
Lin, H., Mosmann, T.R., Guilbert, L., Tuntipopipat, S. & Wegmann, T.G. Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573 (1993).
Piccinni, M.P. et al. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat. Med. 4, 1020–1024 (1998).
Blois, S.M. et al. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the TH1/TH2 cytokine profile. J. Immunol. 172, 5893–5899 (2004).
Aluvihare, V.R., Kallikourdis, M. & Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5, 266–271 (2004).
Kallikourdis, M., Andersen, K.G., Welch, K.A. & Betz, A.G. Alloantigen-enhanced accumulation of CCR5+ 'effector' regulatory T cells in the gravid uterus. Proc. Natl. Acad. Sci. USA 104, 594–599 (2007).
Sasaki, Y. et al. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol. Hum. Reprod. 10, 347–353 (2004).
Somerset, D.A., Zheng, Y., Kilby, M.D., Sansom, D.M. & Drayson, M.T. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology 112, 38–43 (2004).
Zhu, X.Y. et al. Blockade of CD86 signaling facilitates a TH2 bias at the maternal-fetal interface and expands peripheral CD4+CD25+ regulatory T cells to rescue abortion-prone fetuses. Biol. Reprod. 72, 338–345 (2005).
Guleria, I. et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J. Exp. Med. 202, 231–237 (2005).
Poehlmann, T.G. et al. The possible role of the Jak/STAT pathway in lymphocytes at the fetomaternal interface. Chem. Immunol. Allergy 89, 26–35 (2005).
Ayatollahi, M., Geramizadeh, B. & Samsami, A. Transforming growth factor β-1 influence on fetal allografts during pregnancy. Transplant. Proc. 37, 4603–4604 (2005).
Clark, D.A., Banwatt, D. & Chaouat, G. Stress-triggered abortion in mice prevented by alloimmunization. Am. J. Reprod. Immunol. 29, 141–147 (1993).
Blois, S. et al. Intercellular adhesion molecule-1/LFA-1 cross talk is a proximate mediator capable of disrupting immune integration and tolerance mechanism at the feto-maternal interface in murine pregnancies. J. Immunol. 174, 1820–1829 (2005).
Kammerer, U. et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am. J. Pathol. 162, 887–896 (2003).
Miyazaki, S. et al. Predominance of TH2-promoting dendritic cells in early human pregnancy decidua. J. Leukoc. Biol. 74, 514–522 (2003).
Arck, P., Hansen, P.J., Mulac Jericevic, B., Piccinni, M.P. & Szekeres-Bartho, J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 58, 268–279 (2007).
Rabinovich, G.A., Liu, F.T., Hirashima, M. & Anderson, A. An emerging role for galectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand. J. Immunol. 66, 143–158 (2007).
Stillman, B.N. et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 176, 778–789 (2006).
Blaser, C. β-galactoside–binding protein secreted by activated T cells inhibits antigen-induced proliferation of T cells. Eur. J. Immunol. 28, 2311–2319 (1998).
Chung, C.D., Patel, V.P., Moran, M., Lewis, L.A. & Miceli, M.C. Galectin-1 induces partial TCR ζ-chain phosphorylation and antagonizes processive TCR signal transduction. J. Immunol. 165, 3722–3729 (2000).
Stowell, S.R. et al. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood 109, 219–227 (2007).
Rabinovich, G.A. et al. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology 97, 100–106 (1999).
Rabinovich, G.A. et al. Recombinant galectin-1 and its genetic delivery suppress collagen-induced arthritis via T cell apoptosis. J. Exp. Med. 190, 385–398 (1999).
Toscano, M.A. et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant TH2- and T regulatory–mediated anti-inflammatory responses. J. Immunol. 176, 6323–6332 (2006).
Perone, M.J. et al. Dendritic cells expressing transgenic galectin-1 delay onset of autoimmune diabetes in mice. J. Immunol. 177, 5278–5289 (2006).
Rubinstein, N. et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell–mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 5, 241–251 (2004).
von Wolff, M., Wang, X., Gabius, H.J. & Strowitzki, T. Galectin fingerprinting in human endometrium and decidua during the menstrual cycle and in early gestation. Mol. Hum. Reprod. 11, 189–194 (2005).
Phillips, B. et al. Differential expression of two β-galactoside–binding lectins in the reproductive tracts of pregnant mice. Biol. Reprod. 55, 548–558 (1996).
Maquoi, E., van den Brule, F.A., Castronovo, V. & Foidart, J.M. Changes in the distribution pattern of galectin-1 and galectin-3 in human placenta correlates with the differentiation pathways of trophoblasts. Placenta 18, 433–439 (1997).
Bozic, M. et al. Galectin-1 and galectin-3 in the trophoblast of the gestational trophoblastic disease. Placenta 25, 797–802 (2004).
Koopman, L.A. et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J. Exp. Med. 198, 1201–1212 (2003).
Adamson, S.L. et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev. Biol. 250, 358–373 (2002).
Faria, T.N., Ogren, L., Talamantes, F., Linzer, D.I. & Soares, M.J. Localization of placental lactogen-I in trophoblast giant cells of the mouse placenta. Biol. Reprod. 44, 327–331 (1991).
Chaouat, G. et al. A brief review of recent data on some cytokine expressions at the materno-foetal interface which might challenge the classical TH1/TH2 dichotomy. J. Reprod. Immunol. 53, 241–256 (2002).
Toscano, M.A. et al. Differential glycosylation of TH1, TH2 and TH17 effector cells selectively regulates susceptibility to cell death. Nat. Immunol. 8, 825–834 (2007).
Zhu, C. et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6, 1245–1252 (2005).
Choe, Y.S. et al. Expression of galectin-1 mRNA in the mouse uterus is under the control of ovarian steroids during blastocyst implantation. Mol. Reprod. Dev. 48, 261–266 (1997).
Walzel, H. et al. Effects of galectin-1 on regulation of progesterone production in granulosa cells from pig ovaries in vitro. Glycobiology 14, 871–881 (2004).
Jeschke, U. et al. Binding of galectin-1 (Gal-1) on trophoblast cells and inhibition of hormone production of trophoblast tumor cells in vitro by Gal-1. Histochem. Cell Biol. 121, 501–508 (2004).
Erlebacher, A., Zhang, D., Parlow, A.F. & Glimcher, L.H. Ovarian insufficiency and early pregnancy loss induced by activation of the innate immune system. J. Clin. Invest. 114, 39–48 (2004).
Blois, S.M. et al. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol. Reprod. 70, 1018–1023 (2004).
Fulcher, J.A. et al. Galectin-1–matured human monocyte-derived dendritic cells have enhanced migration through extracellular matrix. J. Immunol. 177, 216–226 (2006).
Garcia, M.G. et al. High expression of survivin and down-regulation of Stat-3 characterize the feto-maternal interface in failing murine pregnancies during the implantation period. Placenta 28, 650–657 (2007).
Liu, A.X. et al. Proteomic analysis on the alteration of protein expression in the placental villous tissue of early pregnancy loss. Biol. Reprod. 75, 414–420 (2006).
van der Leij, J. et al. Strongly enhanced IL-10 production using stable galectin-1 homodimers. Mol. Immunol. 44, 506–513 (2007).
Garin, M.I. et al. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood 109, 2058–2065 (2007).
Poirier, F. & Robertson, E.J. Normal development of mice carrying a null mutation in the gene encoding the L14 S-type lectin. Development 119, 1229–1236 (1993).
Acknowledgements
We thank E. Hagen, P. Moschansky and P. Busse for excellent technical assistance in generating this work. Pgr−/− mice were provided by J. Lydon (University of Texas). S.M.B., M.T., U.R.M., J.S.-B. and P.C.A. are part of the Embryo Implantation Control Network of Excellence, co-financed by the European Commission throughout the FP6 framework program Life Science, Genomics and Biotechnology for Health. S.M.B is a fellow of the Habilitation program at the Charité, University Medicine Berlin. J.M.I., M.A.T. and G.A.B. are fellows of the CONICET. A.S.O. is supported by the Turkish Higher Education Council. M.G. was supported by the German Academic Exchange Program. This work was supported by research grants from the German Research Foundation (AR232/8–1, P.C.A.), the Drs. Graute and Graute-Oppermann Foundation (P.C.A.), the Charité (P.C.A.), the Sales Foundation/CONICET Program (G.A.R.), the Mizutani Foundation for Glycoscience (G.A.R.), the Cancer Research Institute (E. Shephard Investigator; G.A.R.), the John Simon Guggenheim Memorial Foundation (G.A.R.), the Argentina National Agency for Promotion of Science and Technology (PICT 2003–05–13787; G.A.R.), the University of Buenos Aires (M091; G.A.R.) and the Association pour la Recherche contre le Cancer and Ligue contre le cancer, comité de Paris (F.P.). We are indebted to S. Cookson, B. Huppertz, D.A. Clark, H.F. Rosenberg and several anonymous reviewers for helpful feedback and constructive comments on this article.
Author information
Authors and Affiliations
Contributions
S.M.B. developed the concept and formulated the key research questions, designed and conducted most of the experiments and wrote the manuscript. J.M.I. and M.T. designed and performed some of the experiments, M.G., A.S.O. and R.C.-R. assisted with experiments presented in Figures 1, 2, 3, 4, M.A.T. and G.A.B. contributed with essential reagents and intellectual input, P.K. assisted with histological analysis and confocal microscopy, B.H. and I.T. assisted with histological and statistical analyses, U.R.M. provided advice in the context of the Stat3 analysis, F.P. provided the Lgals1−/− mice and essential advice, B.F.K. assisted with confocal microscopy and gave input on writing the manuscript, J.S.-B. performed PIBF analysis, and G.A.R. and P.C.A. jointly supervised the work, designed the experiments and wrote the manuscript.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Tables 1–4 and Supplementary Methods (PDF 1982 kb)
Rights and permissions
About this article
Cite this article
Blois, S., Ilarregui, J., Tometten, M. et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med 13, 1450–1457 (2007). https://doi.org/10.1038/nm1680
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1680
This article is cited by
-
The role of galectins in immunity and infection
Nature Reviews Immunology (2023)
-
Phenotyping placental oxygenation in Lgals1 deficient mice using 19F MRI
Scientific Reports (2021)
-
Environmental signals rather than layered ontogeny imprint the function of type 2 conventional dendritic cells in young and adult mice
Nature Communications (2021)
-
Galectin-3 deficiency in pregnancy increases the risk of fetal growth restriction (FGR) via placental insufficiency
Cell Death & Disease (2020)
-
Role of galectin-glycan circuits in reproduction: from healthy pregnancy to preterm birth (PTB)
Seminars in Immunopathology (2020)