Abstract
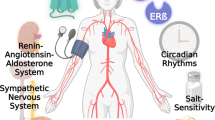
The cardioprotective effects of estrogen are mediated by receptors expressed in vascular cells. Here we show that 27-hydroxycholesterol (27HC), an abundant cholesterol metabolite that is elevated with hypercholesterolemia and found in atherosclerotic lesions, is a competitive antagonist of estrogen receptor action in the vasculature. 27HC inhibited both the transcription-mediated and the non-transcription-mediated estrogen-dependent production of nitric oxide by vascular cells, resulting in reduced estrogen-induced vasorelaxation of rat aorta. Furthermore, increasing 27HC levels in mice by diet-induced hypercholesterolemia, pharmacologic administration or genetic manipulation (by knocking out the gene encoding the catabolic enzyme CYP7B1) decreased estrogen-dependent expression of vascular nitric oxide synthase and repressed carotid artery reendothelialization. As well as antiestrogenic effects, there were proestrogenic actions of 27HC that were cell-type specific, indicating that 27HC functions as an endogenous selective estrogen receptor modulator (SERM). Taken together, these studies point to 27HC as a contributing factor in the loss of estrogen protection from vascular disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Deroo, B.J. & Korach, K.S. Estrogen receptors and human disease. J. Clin. Invest. 116, 561–570 (2006).
Mendelsohn, M.E. & Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 340, 1801–1811 (1999).
Lindner, V. et al. Increased expression of estrogen receptor-beta mRNA in male blood vessels after vascular injury. Circ. Res. 83, 224–229 (1998).
Brouchet, L. et al. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation 103, 423–428 (2001).
Pare, G. et al. Estrogen receptor-alpha mediates the protective effects of estrogen against vascular injury. Circ. Res. 90, 1087–1092 (2002).
Zhu, Y. et al. Abnormal vascular function and hypertension in mice deficient in estrogen receptor beta. Science 295, 505–508 (2002).
Hodgin, J.B. & Maeda, N. Minireview: estrogen and mouse models of atherosclerosis. Endocrinology 143, 4495–4501 (2002).
Mendelsohn, M.E. & Karas, R.H. Molecular and cellular basis of cardiovascular gender differences. Science 308, 1583–1587 (2005).
Colditz, G.A. et al. Menopause and the risk of coronary heart disease in women. N. Engl. J. Med. 316, 1105–1110 (1987).
Turgeon, J.L., McDonnell, D.P., Martin, K.A. & Wise, P.M. Hormone therapy: physiological complexity belies therapeutic simplicity. Science 304, 1269–1273 (2004).
Karas, R.H. Current controversies regarding the cardiovascular effects of hormone therapy. Clin. Obstet. Gynecol. 47, 489–499 (2004).
Hulley, S. et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. J. Am. Med. Assoc. 280, 605–613 (1998).
Rossouw, J.E. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. J. Am. Med. Assoc. 288, 321–333 (2002).
Clark, J.H. A critique of Women's Health Initiative studies (2002–2006). Nucl. Recept. Signal. [online] 4, e023 (2006).
Brown, A.J. & Jessup, W. Oxysterols and atherosclerosis. Atherosclerosis 142, 1–28 (1999).
Schroepfer, G.J., Jr. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol. Rev. 80, 361–554 (2000).
Russell, D.W. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta 1529, 126–135 (2000).
Janowski, B.A. et al. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRβ. Proc. Natl. Acad. Sci. USA 96, 266–271 (1999).
Dzeletovic, S., Breuer, O., Lund, E. & Diczfalusy, U. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225, 73–80 (1995).
Garcia-Cruset, S., Carpenter, K.L., Guardiola, F., Stein, B.K. & Mitchinson, M.J. Oxysterol profiles of normal human arteries, fatty streaks and advanced lesions. Free Radic. Res. 35, 31–41 (2001).
Upston, J.M. et al. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am. J. Pathol. 160, 701–710 (2002).
Crisby, M., Nilsson, J., Kostulas, V., Bjorkhem, I. & Diczfalusy, U. Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim. Biophys. Acta 1344, 278–285 (1997).
Li-Hawkins, J., Lund, E.G., Turley, S.D. & Russell, D.W. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem. 275, 16536–16542 (2000).
Christopherson, K.S. & Bredt, D.S. Nitric oxide in excitable tissues: physiological roles and disease. J. Clin. Invest. 100, 2424–2429 (1997).
Papapetropoulos, A., Rudic, R.D. & Sessa, W.C. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc. Res. 43, 509–520 (1999).
Li, H. & Forstermann, U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 190, 244–254 (2000).
Chambliss, K.L. & Shaul, P.W. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 23, 665–686 (2002).
Takahashi, K. et al. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J. Endocrinol. 178, 319–329 (2003).
Ravi, J., Mantzoros, C.S., Prabhu, A.S., Ram, J.L. & Sowers, J.R. In vitro relaxation of phenylephrine- and angiotensin II-contracted aortic rings by beta-estradiol. Am. J. Hypertens. 7, 1065–1069 (1994).
Parker, M.G., Arbuckle, N., Dauvois, S., Danielian, P. & White, R. Structure and function of the estrogen receptor. Ann. NY Acad. Sci. 684, 119–126 (1993).
Yu, L., Cao, G., Repa, J. & Stangl, H. Sterol regulation of scavenger receptor class B type I in macrophages. J. Lipid Res. 45, 889–899 (2004).
Yuhanna, I.S. et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 7, 853–857 (2001).
Iwakura, A. et al. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation 108, 3115–3121 (2003).
Omoto, Y., Lathe, R., Warner, M. & Gustafsson, J.A. Early onset of puberty and early ovarian failure in CYP7B1 knockout mice. Proc. Natl. Acad. Sci. USA 102, 2814–2819 (2005).
Brzozowski, A.M. et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997).
Shiau, A.K. et al. Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat. Struct. Biol. 9, 359–364 (2002).
Rubanyi, G.M. et al. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J. Clin. Invest. 99, 2429–2437 (1997).
Rosenfeld, M.E. et al. Estrogen inhibits the initiation of fatty streaks throughout the vasculature but does not inhibit intra-plaque hemorrhage and the progression of established lesions in apolipoprotein E deficient mice. Atherosclerosis 164, 251–259 (2002).
Hanke, H. et al. Effect of 17-beta estradiol on pre-existing atherosclerotic lesions: role of the endothelium. Atherosclerosis 147, 123–132 (1999).
Clarkson, T.B. & Appt, S.E. Controversies about HRT–lessons from monkey models. Maturitas 51, 64–74 (2005).
Grodstein, F., Clarkson, T.B. & Manson, J.E. Understanding the divergent data on postmenopausal hormone therapy. N. Engl. J. Med. 348, 645–650 (2003).
Herrington, D.M. et al. Statin therapy, cardiovascular events, and total mortality in the Heart and Estrogen/Progestin Replacement Study (HERS). Circulation 105, 2962–2967 (2002).
Makishima, M. et al. Vitamin D receptor as an intestinal bile acid sensor. Science 296, 1313–1316 (2002).
Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 29, 2905–2919 (2001).
Seetharam, D. et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ. Res. 98, 63–72 (2006).
Couse, J.F., Yates, M.M., Walker, V.R. & Korach, K.S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol. Endocrinol. 17, 1039–1053 (2003).
Repa, J.J. et al. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science 289, 1524–1529 (2000).
Lund, E.G. & Diczfalusy, U. Quantitation of receptor ligands by mass spectrometry. Methods Enzymol. 364, 24–37 (2003).
Bookout, A.L. & Mangelsdorf, D.J. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. [online] 1, e012 (2003).
Acknowledgements
The authors thank Y. Mizuno, K. Kamm, J. Stull and D. Seetharam for help with NOS activity, aortic tension measurement and cell migration assays; C.J.S. Edgell (University of North Carolina) for providing EA.hy926 cells; A. Liverman (University of Texas Southwestern) for aorta samples; D.W. Russell (University of Texas Southwestern) for Cyp7b1−/− mice; and S. Kliewer and members of the Mangelsdorf lab for discussions and suggestions. D.J.M. is an investigator at the Howard Hughes Medical Institute. This work was funded by the Howard Hughes Medical Institute, the Robert A. Welch Foundation (grant I-1275), and US National Institutes of Health grants HL87564 (P.W.S., D.J.M.) and U19DK62434 (D.J.M.).
Author information
Authors and Affiliations
Contributions
M.U. designed, executed and interpreted most of the experiments and prepared the manuscript. H.D. performed initial experiments showing that 27HC is an ER ligand. A.K.G. performed reendothelialization experiments. I.S.Y. performed NOS enzyme assays. C.L.C. designed and performed the LC/MS experiments. N.B.J. synthesized and formulated 27HC. K.S.K. supplied the Esr−/− mice and contributed to the design of the mouse studies. P.W.S. and D.J.M. conceived, planned and supervised the project and wrote the paper.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 642 kb)
Rights and permissions
About this article
Cite this article
Umetani, M., Domoto, H., Gormley, A. et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13, 1185–1192 (2007). https://doi.org/10.1038/nm1641
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1641
This article is cited by
-
Endogenous estrogen receptor modulating oxysterols and breast cancer prognosis: Results from the MARIE patient cohort
British Journal of Cancer (2023)
-
Role of reactive oxygen species in regulating 27-hydroxycholesterol-induced apoptosis of hematopoietic progenitor cells and myeloid cell lines
Cell Death & Disease (2022)
-
The effect of CYP7B1 polymorphisms on the risk of coronary heart disease in Hainan Han population
BMC Medical Genomics (2021)
-
High CYP27A1 expression is a biomarker of favorable prognosis in premenopausal patients with estrogen receptor positive primary breast cancer
npj Breast Cancer (2021)
-
CYP27A1 expression is associated with risk of late lethal estrogen receptor-positive breast cancer in postmenopausal patients
Breast Cancer Research (2020)