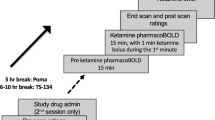
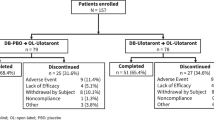
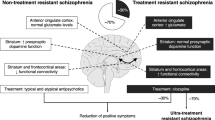
Abstract
Schizophrenia is a chronic, complex and heterogeneous mental disorder, with pathological features of disrupted neuronal excitability and plasticity within limbic structures of the brain. These pathological features manifest behaviorally as positive symptoms (including hallucinations, delusions and thought disorder), negative symptoms (such as social withdrawal, apathy and emotional blunting) and other psychopathological symptoms (such as psychomotor retardation, lack of insight, poor attention and impulse control)1. Altered glutamate neurotransmission has for decades been linked to schizophrenia, but all commonly prescribed antipsychotics act on dopamine receptors2. LY404039 is a selective agonist for metabotropic glutamate 2/3 (mGlu2/3) receptors3 and has shown antipsychotic potential in animal studies. With data from rodents, we provide new evidence that mGlu2/3 receptor agonists work by a distinct mechanism different from that of olanzapine. To clinically test this mechanism, an oral prodrug of LY404039 (LY2140023) was evaluated in schizophrenic patients with olanzapine as an active control in a randomized, three-armed, double-blind, placebo-controlled study. Treatment with LY2140023, like treatment with olanzapine, was safe and well-tolerated; treated patients showed statistically significant improvements in both positive and negative symptoms of schizophrenia compared to placebo (P < 0.001 at week 4). Notably, patients treated with LY2140023 did not differ from placebo-treated patients with respect to prolactin elevation, extrapyramidal symptoms or weight gain. These data suggest that mGlu2/3 receptor agonists have antipsychotic properties and may provide a new alternative for the treatment of schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
17 September 2007
Nat. Med. 13, 1102-1107 (2007); published online 2 September; corrected after print 17 September 2007. In the version of this article initially published, the affiliation of the name of one person acknowledged was misspelled, and reference 4 should have been: Moghaddam, B. & Adams, B.W. Reversal of phenylcyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352. These errors have been corrected in the HTML and PDF versions of the article.
References
Andreasen, N.C. & Carpenter, W.T., Jr. Diagnosis and classification of schizophrenia. Schizophr. Bull. 19, 199–214 (1993).
Tamminga, C.A. & Lahti, A.C. The new generation of antipsychotic drugs. Int. Clin. Psychopharmacol. 11, 73–76 (1996).
Rorick-Kehn, L.M. et al. Pharmacological and pharmacokinetic properties of a structurally-novel, potent, selective mGlu2/3 receptor agonist: in vitro characterization of LY404039. J. Pharmacol. Exp. Ther. 321, 308–317 (2007).
Moghaddam, B. & Adams, B.W. Reversal of phenylcyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science 281, 1349–1352.
Schoepp, D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 299, 12–20 (2001).
Large, C.H., Webster, E.L. & Goff, D.C. The potential role of lamotrigine in schizophrenia. Psychopharmacology (Berl.) 181, 415–436 (2005).
Kremer, I. et al. Placebo-controlled trial of lamotrigine added to conventional and atypical antipsychotics in schizophrenia. Biol. Psychiatry 56, 441–446 (2004).
Tuominen, H.J., Tiihonen, J. & Wahlbeck, K. Glutamatergic drugs for schizophrenia: a systematic review and meta-analysis. Schizophr. Res. 72, 225–234 (2005).
Goff, D.C. et al. A six-month, placebo-controlled trial of D-cycloserine co-administered with conventional antipsychotics in schizophrenia patients. Psychopharmacology (Berl.) 179, 144–150 (2005).
Heresco-Levy, U. et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol. Psychiatry 57, 577–585 (2005).
Schoepp, D.D. & Marek, G.J. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Current Drug Targets. CNS & Neurological Disorders. 1, 215–225 (2002).
Swanson, C.J. & Schoepp, D.D. The group II metabotropic glutamate agonist LY379268 and clozapine reverse phencyclidine-induced behaviors in monoamine-depleted rats. J. Pharmacol. Exp. Ther. 303, 919–927 (2002).
Aghajanian, G.K. & Marek, G.J. Serotonin model of schizophrenia: emerging role of glutamate mechanisms. Brain Research. Brain Res. Brain Res. Rev. 31, 302–312 (2000).
Meltzer, H.Y. Neuropsychopharmacology: The Fifth Generation of Progress (eds. Davis, K.L., Charney, D., Coyle, J.T. & Nemeroff, C.) 819–831 (Lippincott Williams & Wilkins, 2002).
Marek, G.J., Wright, R.A., Schoepp, D.D., Monn, J.A. & Aghajanian, G.K. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 292, 76–87 (2000).
Aghajanian, G.K. & Marek, G.J. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 36, 589–599 (1997).
Marek, G.J., Wright, R.A., Gewirtz, J.C. & Schoepp, D.D. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience 105, 379–392 (2001).
Gewirtz, J.C. & Marek, G.J. Behavioral evidence for interactions between a hallucinogenic drug and group II metabotropic glutamate receptors. Neuropsychopharmacology 23, 569–576 (2000).
Zhai, Y. et al. Group II metabotropic glutamate receptor modulation of DOI-induced c-fos mRNA and excitatory responses in the cerebral cortex. Neuropsychopharmacology 28, 45–52 (2003).
Ellenbroek, B.A. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol. Ther. 57, 1–78 (1993).
Svensson, L., Zhang, J., Johannessen, K. & Engel, J.A. Effect of local infusion of glutamate analogues into the nucleus accumbens of rats: an electrochemical and behavioural study. Brain Res. 643, 155–161 (1994).
Xi, Z.X. et al. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J. Pharmacol. Exp. Ther. 303, 608–615 (2002).
Hu, G., Duffy, P., Swanson, C., Ghasemzadeh, M.B. & Kalivas, P.W. The regulation of dopamine transmission by metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 289, 412–416 (1999).
Cartmell, J., Monn, J.A. & Schoepp, D.D. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus D-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 291, 161–170 (1999).
Flor, P.J., Battaglia, G., Nicoletti, F., Gasparini, F. & Bruno, V. Neuroprotective activity of metabotropic glutamate receptor ligands. Adv. Exp. Med. Biol. 513, 197–223 (2002).
Fleischhacker, W.W., Meise, U., Gunther, V. & Kurz, M. Compliance with antipsychotic drug treatment: influence of side effects. Acta Psychiatr. Scand. Suppl. 382, 11–15 (1994).
Crawford, A.M., Beasley, C.M., Jr. & Tollefson, G.D. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr. Res. 26, 41–54 (1997).
Johnson, M.P. & Chamberlain, M. Modulation of stress-induced and stimulated hyperprolactinemia with the group II metabotropic glutamate receptor selective agonist, LY379268. Neuropharmacology 43, 799–808 (2002).
Kay, S.R., Opler, L.A. & Fiszbein, A. Positive and negative syndrome scale (PANSS) manual. (Multi-Health Systems, North Tonawanda, New York, USA, 1986).
Guy, W. ECDEU assessment manual for psychopharmacology, revised version. (US Department of Health, Education and Welfare, Bethesda, Maryland, USA, 1976).
Acknowledgements
We thank the Lilly Russian Affiliate Team (E. Faustova, N. Jarkova, O. Orlova, O. Larionova, M. Ipatov, M. Putilovsky), the Lilly mGlu2/3 Program Team, R.C. Mohs, X. Peng, P. Cana, S. Marrero, A. Delvaux, J. Ryner, B. Moser, D. Michelson, B. Kinon, A. Stepanov, E. Roberts, I. Pavo, B. Gaydos, D. Manner, F. Wilhite, W.Z. Potter, C.M. Beasley and S.M. Paul.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.T.P., L.Z., F.M., S.L.L., K.A.J., J.A.M., B.G.J., & D.D.S. are/were employees of Eli Lilly and Company at the time of this clinical study. Several of them own Lilly stock.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–4, Supplementary Figs. 1–3, Supplementary Methods (PDF 141 kb)
Rights and permissions
About this article
Cite this article
Patil, S., Zhang, L., Martenyi, F. et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13, 1102–1107 (2007). https://doi.org/10.1038/nm1632
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1632
This article is cited by
-
Effects of the mGlu2/3 receptor agonist LY379268 on two models of disturbed auditory evoked brain oscillations in mice
Translational Psychiatry (2023)
-
Distribution of [11C]-JNJ-42491293 in the marmoset brain: a positron emission tomography study
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
The mGluR2/3 orthosteric agonist LY-404,039 reduces dyskinesia, psychosis-like behaviours and parkinsonism in the MPTP-lesioned marmoset
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Effect of mGluR2 positive allosteric modulation on frontostriatal working memory activation in schizophrenia
Molecular Psychiatry (2022)
-
Glutamatergic dysfunction in Schizophrenia
Translational Psychiatry (2022)