Abstract
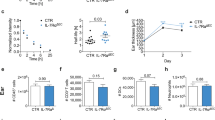
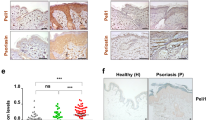
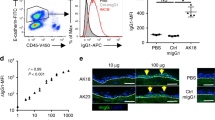
Psoriasis is a common T cell–mediated autoimmune inflammatory disease. We show that blocking the interaction of α1β1 integrin (VLA-1) with collagen prevented accumulation of epidermal T cells and immunopathology of psoriasis. α1β1 integrin, a major collagen-binding surface receptor, was exclusively expressed by epidermal but not dermal T cells. α1β1-positive T cells showed characteristic surface markers of effector memory cells and contained high levels of interferon-γ but not interleukin-4. Blockade of α1β1 inhibited migration of T cells into the epidermis in a clinically relevant xenotransplantation model. This was paralleled by a complete inhibition of psoriasis development, comparable to that caused by tumor necrosis factor-α blockers. These results define a crucial role for α1β1 in controlling the accumulation of epidermal type 1 polarized effector memory T cells in a common human immunopathology and provide the basis for new strategies in psoriasis treatment focusing on T cell–extracellular matrix interactions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Griffiths, C.E. & Voorhees, J.J. Psoriasis, T cells and autoimmunity. J. R. Soc. Med. 89, 315–319 (1996).
Davidson, A. & Diamond, B. Autoimmune diseases. N. Engl. J. Med. 345, 340–350 (2001).
Kupper, T.S. & Fuhlbrigge, R.C. Immune surveillance in the skin: mechanisms and clinical consequences. Nat. Rev. Immunol. 4, 211–222 (2004).
Nickoloff, B.J. & Nestle, F.O. Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113, 1664–1675 (2004).
Bowcock, A.M. & Krueger, J.G. Getting under the skin: the immunogenetics of psoriasis. Nat. Rev. Immunol. 5, 699–711 (2005).
Schon, M.P. & Boehncke, W.H. Psoriasis. N. Engl. J. Med. 352, 1899–1912 (2005).
Chang, J.C. et al. CD8+ T cells in psoriatic lesions preferentially use T-cell receptor V beta 3 and/or V beta 13.1 genes. Proc. Natl. Acad. Sci. USA 91, 9282–9286 (1994).
Krueger, J.G. et al. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J. Exp. Med. 182, 2057–2068 (1995).
Chamian, F. et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc. Natl. Acad. Sci. USA 102, 2075–2080 (2005).
Baker, B.S., Swain, A.F., Fry, L. & Valdimarsson, H. Epidermal T lymphocytes and HLA-DR expression in psoriasis. Br. J. Dermatol. 110, 555–564 (1984).
Austin, L.M., Ozawa, M., Kikuchi, T., Walters, I.B. & Krueger, J.G. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. J. Invest. Dermatol. 113, 752–759 (1999).
Boyman, O. et al. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-α. J. Exp. Med. 199, 731–736 (2004).
Boyman, O., Conrad, C., Tonel, G., Gilliet, M. & Nestle, F.O. The pathogenic role of tissue-resident immune cells in psoriasis. Trends Immunol. 28, 51–57 (2007).
Ghohestani, R.F., Li, K., Rousselle, P. & Uitto, J. Molecular organization of the cutaneous basement membrane zone. Clin. Dermatol. 19, 551–562 (2001).
Bosman, F.T. & Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 200, 423–428 (2003).
Hemler, M.E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu. Rev. Immunol. 8, 365–400 (1990).
Hynes, R.O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
deFougerolles, A.R. Integrins in immune and inflammatory disease. In I Domains in Integrins (ed. Gullberg, D.) 165–184 (Landes Biosciences, Austin, Texas, USA, 2003).
Ben-Horin, S. & Bank, I. The role of very late antigen-1 in immune-mediated inflammation. Clin. Immunol. 113, 119–129 (2004).
Ray, S.J. et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179 (2004).
Fiorucci, S. et al. Importance of innate immunity and collagen binding integrin α1β1 in TNBS-induced colitis. Immunity 17, 769–780 (2002).
Chaudhari, U. et al. Efficacy and safety of infliximab monotherapy for plaque-type psoriasis: a randomised trial. Lancet 357, 1842–1847 (2001).
Reich, K. et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366, 1367–1374 (2005).
Boehncke, W.H., Kellner, I., Konter, U. & Sterry, W. Differential expression of adhesion molecules on infiltrating cells in inflammatory dermatoses. J. Am. Acad. Dermatol. 26, 907–913 (1992).
Pauls, K. et al. Role of integrin αE(CD103)β7 for tissue-specific epidermal localization of CD8+ T lymphocytes. J. Invest. Dermatol. 117, 569–575 (2001).
Teraki, Y. & Shiohara, T. Preferential expression of αEβ7 integrin (CD103) on CD8+ T cells in the psoriatic epidermis: regulation by interleukins 4 and 12 and transforming growth factor-β. Br. J. Dermatol. 147, 1118–1126 (2002).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22, 745–763 (2004).
Selin, L.K. & Cornberg, M. Embedding T cells in the matrix. Nat. Med. 10, 343–345 (2004).
Goldstein, I. et al. Expression of the α1β1 integrin, VLA-1, marks a distinct subset of human CD4+ memory T cells. J. Clin. Invest. 112, 1444–1454 (2003).
Christophers, E., Parzefall, R. & Braun-Falco, O. Initial events in psoriasis: quantitative assessment. Br. J. Dermatol. 89, 327–334 (1973).
Ragaz, A. & Ackerman, A.B. Evolution, maturation, and regression of lesions of psoriasis. New observations and correlation of clinical and histologic findings. Am. J. Dermatopathol. 1, 199–214 (1979).
Gotwals, P.J. et al. Divalent cations stabilize the α1β1 integrin I domain. Biochemistry 38, 8280–8288 (1999).
Krueger, J.G. & Bowcock, A. Psoriasis pathophysiology: current concepts of pathogenesis. Ann. Rheum. Dis. 64 (suppl. 2), ii30–ii36 (2005).
Nestle, F.O. et al. Plasmacytoid pre-dendritic cells (PDC) initiate psoriasis through IFN-alpha production. J. Exp. Med. 202, 135–143 (2005).
Nestle, F.O. & Gilliet, M. Defining upstream elements of psoriasis pathogenesis: an emerging role for interferon alpha. J. Invest. Dermatol. 125, xiv–xv (2005).
Boyman, O. et al. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 152, 1211–1218 (2005).
Hayday, A., Theodoridis, E., Ramsburg, E. & Shires, J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat. Immunol. 2, 997–1003 (2001).
Fraki, J.E., Briggaman, R.A. & Lazarus, G.S. Transplantation of psoriatic skin onto nude mice. J. Invest. Dermatol. 80 (suppl.), 31s–35s (1983).
Clark, R.A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Acknowledgements
We thank B.J. Nickoloff, M. Gilliet, K.S. Lang and P. Johansen for discussions. We appreciate the excellent technical assistance by C. Dudli and A. Perez. We would further like to thank T. Baechi for help with confocal microscopy. This work was supported by the Swiss National Science Foundation, Biogen-Idec, the Bonizzi-Theler-Foundation and the Wellcome Trust. The authors acknowledge financial support from the UK Department of Health through the National Institute for Health Research Biomedical Research Centre award to Guy's & St Thomas' National Health Service Foundation Trust in partnership with King's College London.
Author information
Authors and Affiliations
Contributions
C.C., O.B. and G.T. performed mouse, cell culture and molecular biology experiments and data analysis. A.T.-K. performed PCR experiments and data analysis. U.L. performed flow cytometry, culture of cell lines and clones and data analysis. A.d.F., V.K. and H.G. provided expertise, reagents and input into manuscript preparation. Experimental design, data analysis and manuscript preparation were carried out by C.C. and F.O.N. All authors read and commented on the paper.
Corresponding authors
Ethics declarations
Competing interests
A.d.F., V.K. and H.G. are former employees of Biogen Idec.
F.O.N. has been consulting for and received lecturing fees from Biogen Idec.
The study has received scientific grant support from Biogen Idec.
Supplementary information
Supplementary Fig. 1
CD3+/CD8+ and CD3+/CD4+ epidermal T cells express α1 integrin. (PDF 133 kb)
Supplementary Fig. 2
Extended phenotype of freshly isolated α1β1+ psoriatic T cells. (PDF 120 kb)
Supplementary Fig. 3
Extended phenotype of α1β1 expressing psoriatic T cell lines. (PDF 269 kb)
Supplementary Fig. 4
Extended phenotype of α1β1 expressing psoriatic T cell clones. (PDF 145 kb)
Supplementary Fig. 5
Efficacy of α1β1 blockade in treatment of established psoriatic skin. (PDF 62 kb)
Supplementary Fig. 6
No induction of apoptosis via monoclonal antibody to α1. (PDF 115 kb)
Supplementary Fig. 7
Migration of T cells through collagen type IV increases α1β1-expression on T cells in vitro. (PDF 34 kb)
Rights and permissions
About this article
Cite this article
Conrad, C., Boyman, O., Tonel, G. et al. α1β1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 13, 836–842 (2007). https://doi.org/10.1038/nm1605
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1605
This article is cited by
-
Disease modification in inflammatory skin disorders: opportunities and challenges
Nature Reviews Drug Discovery (2023)
-
Role of Pro-inflammatory and Anti-inflammatory Cytokines in Pathophysiology of Psoriasis
Current Oral Health Reports (2022)
-
Fractional laser ablation for the targeted cutaneous delivery of an anti-CD29 monoclonal antibody – OS2966
Scientific Reports (2019)
-
TNF blockade induces a dysregulated type I interferon response without autoimmunity in paradoxical psoriasis
Nature Communications (2018)
-
Skin Immunity
Archivum Immunologiae et Therapiae Experimentalis (2018)