Abstract
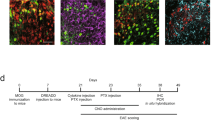
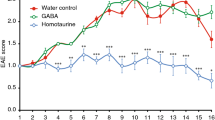
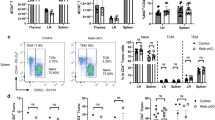
The cannabinoid system is immunomodulatory and has been targeted as a treatment for the central nervous system (CNS) autoimmune disease multiple sclerosis. Using an animal model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE), we investigated the role of the CB1 and CB2 cannabinoid receptors in regulating CNS autoimmunity. We found that CB1 receptor expression by neurons, but not T cells, was required for cannabinoid-mediated EAE suppression. In contrast, CB2 receptor expression by encephalitogenic T cells was critical for controlling inflammation associated with EAE. CB2-deficient T cells in the CNS during EAE exhibited reduced levels of apoptosis, a higher rate of proliferation and increased production of inflammatory cytokines, resulting in severe clinical disease. Together, our results demonstrate that the cannabinoid system within the CNS plays a critical role in regulating autoimmune inflammation, with the CNS directly suppressing T-cell effector function via the CB2 receptor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hickey, W.F., Hsu, B.L. & Kimura, H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 28, 254–260 (1991).
Sospedra, M. & Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23, 683–747 (2005).
Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. & Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564 (1990).
Munro, S., Thomas, K.L. & Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65 (1993).
Kaminski, N.E., Abood, M.E., Kessler, F.K., Martin, B.R. & Schatz, A.R. Identification of a functionally relevant cannabinoid receptor on mouse spleen cells that is involved in cannabinoid-mediated immune modulation. Mol. Pharmacol. 42, 736–742 (1992).
Klein, T.W. et al. The cannabinoid system and immune modulation. J. Leukoc. Biol. 74, 486–496 (2003).
Van Sickle, M.D. et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332 (2005).
Salzet, M., Breton, C., Bisogno, T. & Di Marzo, V. Comparative biology of the endocannabinoid system possible role in the immune response. Eur. J. Biochem. 267, 4917–4927 (2000).
Pertwee, R.G. Cannabinoids and multiple sclerosis. Pharmacol. Ther. 95, 165–174 (2002).
Lyman, W.D., Sonett, J.R., Brosnan, C.F., Elkin, R. & Bornstein, M.B. Δ9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 23, 73–81 (1989).
Wirguin, I. et al. Suppression of experimental autoimmune encephalomyelitis by cannabinoids. Immunopharmacology 28, 209–214 (1994).
Arévalo-Martín, A., Vela, J.M., Molina-Holgado, E., Borrell, J. & Guaza, C. Therapeutic action of cannabinoids in a murine model of multiple sclerosis. J. Neurosci. 23, 2511–2516 (2003).
Pryce, G. et al. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126, 2191–2202 (2003).
Rinaldi-Carmona, M. et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 284, 644–650 (1998).
Rinaldi-Carmona, M. et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 350, 240–244 (1994).
Do, Y., McKallip, R.J., Nagarkatti, M. & Nagarkatti, P.S. Activation through cannabinoid receptors 1 and 2 on dendritic cells triggers NF-κB-dependent apoptosis: novel role for endogenous and exogenous cannabinoids in immunoregulation. J. Immunol. 173, 2373–2382 (2004).
Parolaro, D., Massi, P., Rubino, T. & Monti, E. Endocannabinoids in the immune system and cancer. Prostaglandins Leukot. Essent. Fatty Acids 66, 319–332 (2002).
Ponomarev, E.D. et al. γδ T cell regulation of IFN-γ production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J. Immunol. 173, 1587–1595 (2004).
Ponomarev, E.D. et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J. Immunol. 178, 39–48 (2007).
Sugiura, T. & Waku, K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem. Phys. Lipids 108, 89–106 (2000).
Maresz, K., Carrier, E.J., Ponomarev, E.D., Hillard, C.J. & Dittel, B.N. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J. Neurochem. 95, 437–445 (2005).
Witting, A., Walter, L., Wacker, J., Moller, T. & Stella, N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl. Acad. Sci. USA 101, 3214–3219 (2004).
Witting, A. et al. Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proc. Natl. Acad. Sci. USA 103, 6362–6367 (2006).
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Tronche, F. et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99–103 (1999).
Hennet, T., Hagen, F.K., Tabak, L.A. & Marth, J.D. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc. Natl. Acad. Sci. USA 92, 12070–12074 (1995).
Dittel, B.N., Merchant, R.M. & Janeway, C.A., Jr Evidence for Fas-dependent and Fas-independent mechanisms in the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 162, 6392–6400 (1999).
Buckley, N.E. et al. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB2 receptor. Eur. J. Pharmacol. 396, 141–149 (2000).
Ponomarev, E.D. & Dittel, B.N. γδ T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J. Immunol. 174, 4678–4687 (2005).
Huffman, J.W. et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg. Med. Chem. 7, 2905–2914 (1999).
Acknowledgements
We thank S. Morris-Islo for assistance with the mice and Roche Palo Alto for providing CB1−/− mice. This work was supported in part by US National Institutes of Health grants R01 NS046662 (B.N.D.), R01 NS041314 (C.J.H.) and DA09155 (C.J.H.), the BloodCenter Research Foundation (B.N.D.), the Multiple Sclerosis Society of Great Britain and Northern Ireland, the National Multiple Sclerosis Society and Aims2cure. J.L.C. is a Research Fellow of the Japan Society for the Promotion of Science (P03581). The authors thank the National Institute on Drug Abuse chemical supply program for donating chemicals for this study.
Author information
Authors and Affiliations
Contributions
K.M. helped design and performed all of the experiments depicted in Figures 1 and 2 (except Fig. 1a) and Table 2, with technical advice and assistance from E.D.P., X.C., E.J.C. and M.K.M in B.N.D.'s laboratory. L.P.S. performed the immunohistology in B.N.D.'s laboratory. G.P. bred and screened conditional knockouts and designed and performed EAE experiments. B.L. supervised G.M. in the production of the conditional CB1 floxed mice, obtained the funding and provided the techniques for screening the mice. J.L.C. performed EAE experiments in T.Y. and D.B.'s laboratories, and performed supportive experiments of cannabinoids in EAE, cytokine analysis and T-cell responses in T.Y.'s laboratory. C.L. produced the generalized CB1 receptor knockout mouse and provided techniques for their screening. G.G. assisted with intellectual direction and experimental design and obtained funding for the studies and personnel costs. R.G.P. is a cannabinoid pharmacologist who collaborated on the project since its initiation in 1989 providing intellectual input for experimental design, drug selection and obtained compounds for the project. T.Y. obtained funding for the project. N.E.B. provided the CB2−/− mice. C.J.H. helped with experimental design and writing of the manuscript; her laboratory backcrossed the CB1−/− mice onto the ICR background. D.B. bred and screened conditional mice, initiated the project and performed and designed EAE experiments (Fig. 1a and Table 1). B.N.D. supervised K.M. and colleagues and helped with experimental design. D.B. and B.N.D. secured funding and permission to undertake the study and helped write the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.B. is the founding member of Canbex, a company that aims to produce pharmaceutical cannabinoids for the treatment of multiple sclerosis. R.G.P. is Director of Pharmacology for GW Pharmaceuticals, which produces cannabis-based medicines.
Supplementary information
Supplementary Table 1
Comparison of EAE clinical disease parameters induced by active immunization in WT and CB2−/− mice. (PDF 42 kb)
Rights and permissions
About this article
Cite this article
Maresz, K., Pryce, G., Ponomarev, E. et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med 13, 492–497 (2007). https://doi.org/10.1038/nm1561
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1561
This article is cited by
-
Role of the endocannabinoid system in the pathophysiology of endometriosis and therapeutic implications
Journal of Cannabis Research (2022)
-
Cannabis consumption is associated with lower COVID-19 severity among hospitalized patients: a retrospective cohort analysis
Journal of Cannabis Research (2022)
-
Cannabinoids and the expanded endocannabinoid system in neurological disorders
Nature Reviews Neurology (2020)
-
Gene Expression of ABHD6, a Key Factor in the Endocannabinoid System, Can Be Modulated by Female Hormones in Human Immune Cells
Biochemical Genetics (2019)
-
Cannabinoid CB2 receptors in the mouse brain: relevance for Alzheimer’s disease
Journal of Neuroinflammation (2018)