Abstract
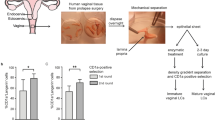
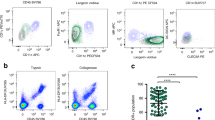
Human immunodeficiency virus-1 (HIV-1) is primarily transmitted sexually. Dendritic cells (DCs) in the subepithelium transmit HIV-1 to T cells through the C-type lectin DC-specific intercellular adhesion molecule (ICAM)-3-grabbing nonintegrin (DC-SIGN). However, the epithelial Langerhans cells (LCs) are the first DC subset to encounter HIV-1. It has generally been assumed that LCs mediate the transmission of HIV-1 to T cells through the C-type lectin Langerin, similarly to transmission by DC-SIGN on dendritic cells (DCs). Here we show that in stark contrast to DC-SIGN, Langerin prevents HIV-1 transmission by LCs. HIV-1 captured by Langerin was internalized into Birbeck granules and degraded. Langerin inhibited LC infection and this mechanism kept LCs refractory to HIV-1 transmission; inhibition of Langerin allowed LC infection and subsequent HIV-1 transmission. Notably, LCs also inhibited T-cell infection by viral clearance through Langerin. Thus Langerin is a natural barrier to HIV-1 infection, and strategies to combat infection must enhance, preserve or, at the very least, not interfere with Langerin expression and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lederman, M.M., Offord, R.E. & Hartley, O. Nat. Rev. Immunol. 6, 371–382 (2006).
Geijtenbeek, T.B. et al. Cell 100, 587–597 (2000).
Turville, S.G. et al. Blood 103, 2170–2179 (2004).
Turville, S.G. et al. Nat. Immunol. 3, 975–983 (2002).
Veazey, R.S. et al. Nature 438, 99–102 (2005).
Patterson, B.K. et al. Am. J. Pathol. 161, 867–873 (2002).
Kawamura, T., Kurtz, S.E., Blauvelt, A. & Shimada, S. J. Dermatol. Sci. 40, 147–155 (2005).
Pope, M. et al. Cell 78, 389–398 (1994).
Reece, J.C. et al. J. Exp. Med. 187, 1623–1631 (1998).
Kawamura, T. et al. Proc. Natl. Acad. Sci. USA 100, 8401–8406 (2003).
Kawamura, T. et al. J. Exp. Med. 192, 1491–1500 (2000).
Collins, K.B., Patterson, B.K., Naus, G.J., Landers, D.V. & Gupta, P. Nat. Med. 6, 475–479 (2000).
Banchereau, J. & Steinman, R.M. Nature 392, 245–252 (1998).
McDonald, D. et al. Science 300, 1295–1297 (2003).
Valladeau, J. et al. Immunity 12, 71–81 (2000).
Hunger, R.E. et al. J. Clin. Invest. 113, 701–708 (2004).
Masterson, A.J. et al. Blood 100, 701–703 (2002).
Garcia, E. et al. Traffic 6, 488–501 (2005).
Richters, C.D. et al. Clin. Exp. Immunol. 98, 330–336 (1994).
Nguyen, D.G. & Hildreth, J.E. Eur. J. Immunol. 33, 483–493 (2003).
Kawamura, T., Qualbani, M., Thomas, E.K., Orenstein, J.M. & Blauvelt, A. Eur. J. Immunol. 31, 360–368 (2001).
Miller, C.J. & Hu, J. J. Infect. Dis. 179(suppl. 3), 413–417 (1999).
Ward, E.M., Stambach, N.S., Drickamer, K. & Taylor, M.E. J. Biol. Chem. 281, 15450–15456 (2006).
Cohn, M.A. et al. J. Infect. Dis. 184, 410–417 (2001).
Picut, C.A., Lee, C.S., Dougherty, E.P., Andersen, K.L. & Lewis, R.M. J. Histochem. Cytochem. 35, 745–753 (1987).
Acknowledgements
We thank L. Colledge, R. Mebius and M. Litjens for their comments on the manuscript, S. Santegoets for helping to set up the MUTZ3 culture, P. Gallay (Scripps Research Institute) for providing us with the pseudotyped HIV-1 viruses and S. Saeland (Schering Plough) for the Langerin plasmid. We are grateful to the Boerhaave Clinic for providing us with essential materials. We thank E.-C. Park and B. Seed (US National Institutes of Health AIDS Research and Reference Reagent Program) for providing the pSyn gp120 IgG reagent. L.d.W., A.N. and M.A.W.P.d.J. were supported by grants from the Dutch Scientific Research program (VIDI NWO 917-46-367; NWO 912-04-025); A.N. was also supported by the Dutch AIDS Foundation (20005033).
Author information
Authors and Affiliations
Contributions
L.d.W. designed, executed and interpreted most experiments and prepared the manuscript. A.N. generated viruses and helped with several experiments. M.P. generated the Langerin lentiviral construct under supervision of V.P., who also helped with the manuscript preparation. D.F. executed and interpreted the electron microscopy analysis. M.A.W.P.d.J. helped with LC isolations. T.d.G. contributed reagents and knowledge on LC isolation. Y.v.K. provided supervision and helped with the manuscript preparation. T.B.H.G. supervised all aspects of this study including study design, execution and interpretation, and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
LCs are a natural barrier against HIV-1 transmission. (PDF 835 kb)
Supplementary Fig. 2
Immature LCs inhibit T cell infection. (PDF 162 kb)
Supplementary Fig. 3
The novel HIV-1 blocking antibody 10E2 stains Langerin on LCs in situ. (PDF 858 kb)
Supplementary Fig. 4
Primary emigrant LCs resemble immature isolated LCs. (PDF 177 kb)
Supplementary Fig. 5
Langerin binds HIV-1 gp120 and inhibits LC infection. (PDF 836 kb)
Supplementary Fig. 6
Lewis X is a potential microbicide that blocks HIV-1 gp120 binding to DC-SIGN, but not Langerin. (PDF 101 kb)
Supplementary Table 1
Summary of data obtained with different donors for LC isolation (PDF 8 kb)
Rights and permissions
About this article
Cite this article
de Witte, L., Nabatov, A., Pion, M. et al. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat Med 13, 367–371 (2007). https://doi.org/10.1038/nm1541
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1541
This article is cited by
-
Immune activation of vaginal human Langerhans cells increases susceptibility to HIV-1 infection
Scientific Reports (2023)
-
Receptor basis of biological activity of polysaccharides
Biophysical Reviews (2023)
-
HIV transmitting mononuclear phagocytes; integrating the old and new
Mucosal Immunology (2022)
-
CGRP inhibits human Langerhans cells infection with HSV by differentially modulating specific HSV-1 and HSV-2 entry mechanisms
Mucosal Immunology (2022)
-
Targeted disruption of pi–pi stacking in Malaysian banana lectin reduces mitogenicity while preserving antiviral activity
Scientific Reports (2021)