Abstract
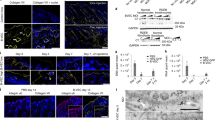
The continuous renewal of human epidermis is sustained by stem cells contained in the epidermal basal layer and in hair follicles1,2. Cultured keratinocyte stem cells, known as holoclones3,4,5,6, generate sheets of epithelium used to restore severe skin, mucosal and corneal defects7,8,9. Mutations in genes encoding the basement membrane component laminin 5 (LAM5) cause junctional epidermolysis bullosa (JEB), a devastating and often fatal skin adhesion disorder10. Epidermal stem cells from an adult patient affected by LAM5-β3–deficient JEB were transduced with a retroviral vector expressing LAMB3 cDNA (encoding LAM5-β3), and used to prepare genetically corrected cultured epidermal grafts. Nine grafts were transplanted onto surgically prepared regions of the patient's legs. Engraftment was complete after 8 d. Synthesis and proper assembly of normal levels of functional LAM5 were observed, together with the development of a firmly adherent epidermis that remained stable for the duration of the follow-up (1 year) in the absence of blisters, infections, inflammation or immune response. Retroviral integration site analysis indicated that the regenerated epidermis is maintained by a defined repertoire of transduced stem cells. These data show that ex vivo gene therapy of JEB is feasible and leads to full functional correction of the disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Watt, F.M. Stem cell fate and patterning in mammalian epidermis. Curr. Opin. Genet. Dev. 11, 410–417 (2001).
Alonso, L. & Fuchs, E. Stem cells of the skin epithelium. Proc. Natl. Acad. Sci. USA 100 (suppl. 1), 11830–11835 (2003).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. USA 84, 2302–2306 (1987).
Rochat, A., Kobayashi, K. & Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 76, 1063–1073 (1994).
Pellegrini, G. et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 145, 769–782 (1999).
Blanpain, C., Lowry, W.E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Gallico, G.G., III, O'Connor, N.E., Compton, C.C., Kehinde, O. & Green, H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N. Engl. J. Med. 311, 448–451 (1984).
Romagnoli, G. et al. Treatment of posterior hypospadias by the autologous graft of cultured urethral epithelium. N. Engl. J. Med. 323, 527–530 (1990).
Pellegrini, G. et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet 349, 990–993 (1997).
Christiano, A.M. & Uitto, J. Molecular complexity of the cutaneous basement membrane zone. Revelations from the paradigms of epidermolysis bullosa. Exp. Dermatol. 5, 1–11 (1996).
Posteraro, P. et al. Compound heterozygosity for an out-of-frame deletion and a splice site mutation in the LAMB3 gene causes nonlethal junctional epidermolysis bullosa. Biochem. Biophys. Res. Commun. 243, 758–764 (1998).
Guerra, L. et al. Treatment of “stable” vitiligo by Timedsurgery and transplantation of cultured epidermal autografts. Arch. Dermatol. 136, 1380–1389 (2000).
Mills, A.A. et al. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature 398, 708–713 (1999).
Yang, A. et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature 398, 714–718 (1999).
Parsa, R., Yang, A., McKeon, F. & Green, H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Invest. Dermatol. 113, 1099–1105 (1999).
Pellegrini, G. et al. p63 identifies keratinocyte stem cells. Proc. Natl. Acad. Sci. USA 98, 3156–3161 (2001).
Di Iorio, E. et al. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc. Natl. Acad. Sci. USA 102, 9523–9528 (2005).
Parker, K.C., Bednarek, M.A. & Coligan, J.E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152, 163–175 (1994).
Manici, S. et al. Melanoma cells present a MAGE-3 epitope to CD4+ cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J. Exp. Med. 189, 871–876 (1999).
Pellegrini, G. et al. The control of epidermal stem cells (holoclones) in the treatment of massive full-thickness burns with autologous keratinocytes cultured on fibrin. Transplantation 68, 868–879 (1999).
Bushman, F. et al. Genome-wide analysis of retroviral DNA integration. Nat. Rev. Microbiol. 3, 848–858 (2005).
De Luca, M., Pellegrini, G. & Green, H. Regeneration of squamous epithelia from stem cells of cultured grafts. Regenerative Med. 1, 45–57 (2006).
Hacein-Bey-Abina, S. et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302, 415–419 (2003).
Gaspar, H.B. et al. Gene therapy of X-linked severe combined immunodeficiency by use of a pseudotyped gammaretroviral vector. Lancet 364, 2181–2187 (2004).
Aiuti, A. et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 (2002).
Recchia, A. et al. Retroviral vector integration deregulates gene expression but has no consequence on the biology and function of transplanted T cells. Proc. Natl. Acad. Sci. USA 103, 1457–1462 (2006).
Ott, M.G. et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1–EVI1, PRDM16 or SETBP1. Nat. Med. 12, 401–409 (2006).
Dellambra, E. et al. Corrective transduction of human epidermal stem cells in laminin-5-dependent junctional epidermolysis bullosa. Hum. Gene Ther. 9, 1359–1370 (1998).
Mathor, M.B. et al. Clonal analysis of stably transduced human epidermal stem cells in culture. Proc. Natl. Acad. Sci. USA 93, 10371–10376 (1996).
Krall, W.J. et al. Increased levels of spliced RNA account for augmented expression from the MFG retroviral vector in hematopoietic cells. Gene Ther. 3, 37–48 (1996).
Acknowledgements
We thank S. Bondanza for the clonal analysis of KEP25 keratinocytes shown in Figure 1b, K. Fleishauer for the HLA genotyping, and C. Rossi and D. Sartori for technical assistance. We also thank H. Green (Harvard Medical School) for providing the 3T3-J2 cells and G. Meneguzzi (Institut National de la Santé et de la Recherche Médicale (INSERM) U 634) for providing the K140 antibody to LAM5-β3 and performing the immunofluorescence shown in Figure 3d. This work was supported by grants from Telethon, AFM-Telethon and the European Commission (VI Framework Program, SKINTHERAPY).
Author information
Authors and Affiliations
Contributions
F.M., G.P. and M.D.L. designed and directed the study. G.P carried out the clonal analysis, transduced the patient's cells and prepared the skin implants. S.F, F.D.N., E.D.I. and G.M. carried out the hystological and molecular follow-up, G.F. and F.M. constructed the retroviral vectors and the packaging cell line, E.P. and C.B. performed the immunological analysis, S.C., A.C., C.M. and A.G. carried out the transplantation and were responsible for the clinical management of the patient.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Regeneration of a genetically corrected epidermis. (PDF 605 kb)
Supplementary Fig. 2
Integration site analysis in cultured keratinocytes. (PDF 59 kb)
Supplementary Table 1
Complete list of retroviral integration sites in skin biopsies 1 and 4 months after transplantation (PDF 85 kb)
Supplementary Table 2
Analysis of retroviral integration sites in skin biopsies (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Mavilio, F., Pellegrini, G., Ferrari, S. et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med 12, 1397–1402 (2006). https://doi.org/10.1038/nm1504
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1504
This article is cited by
-
Netherton syndrome in a Bulgarian patient
Wiener Medizinische Wochenschrift (2023)
-
New developments in the molecular treatment of ichthyosis: review of the literature
Orphanet Journal of Rare Diseases (2022)
-
Mesenchymal Stem Cells From Mouse Hair Follicles Reduce Hypertrophic Scarring in a Murine Wound Healing Model
Stem Cell Reviews and Reports (2022)
-
Eye Involvement and Management in Inherited Epidermolysis Bullosa
Drugs (2022)
-
Epidermolysis Bullosa: A Review of the Tissue-Engineered Skin Substitutes Used to Treat Wounds
Molecular Diagnosis & Therapy (2022)