Abstract
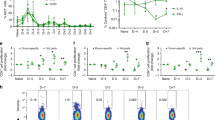
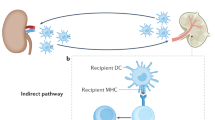
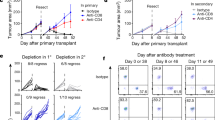
Although major histocompatibility complex (MHC) class II–restricted CD4 T cells are well appreciated for their contribution to peripheral tolerance to tissue allografts, little is known regarding MHC class I–dependent reactivity in this process. Here we show a crucial role for host MHC class I–dependent NK cell reactivity for allograft tolerance in mice induced through either costimulation blockade using CD154-specific antibody therapy or by targeting LFA-1 (also known as CD11a). Tolerance induction absolutely required host expression of MHC class I, but was independent of CD8 T cell–dependent immunity. Rather, tolerance required innate immunity involving NK1.1+ cells, but was independent of CD1d-restricted NKT cells. Therefore, NK cells seem to be generally required for induction of tolerance to islet allografts. Additional studies indicate that CD154-specific antibody–induced allograft tolerance is perforin dependent. Notably, NK cells that are perforin competent are sufficient to restore allograft tolerance in perforin-deficient recipients. Together, these results show an obligatory role for NK cells, through perforin, for induction of tolerance to islet allografts.
Note: In the version of this article initially published, the authors inadvertently misquoted a study as evidence that mouse NKT cells can express CD154 (ref. 29). Rather, the cited study concerned CD40-CD40L interactions in human NK cells. By misquoting this study, the authors also omitted an appropriate reference regarding prior evidence of CD40-CD40L interactions by murine NKT cells (Kitamura, H. et al., J. Exp. Med. 189, 1121; 1999). These errors have been corrected in the PDF version.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
24 February 2006
Note: In the version of this article initially published, the authors inadvertently misquoted a study as evidence that mouse NKT cells can express CD154 (ref. 29). Rather, the cited study concerned CD40-CD40L interactions in human NK cells. By misquoting this study, the authors also omitted an appropriate reference regarding prior evidence of CD40-CD40L interactions by murine NKT cells (Kitamura, H. et al., J. Exp. Med. 189, 1121; 1999). These errors have been corrected in the PDF version.
References
Wood, K.J. & Sakaguchi, S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 3, 199–210 (2003).
Gilliet, M. & Liu, Y. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195, 695–704 (2002).
Zhou, J., Carr, R.I., Liwski, R.S., Stadnyk, A.W. & Lee, T.D. Oral exposure to alloantigen generates intragraft CD8+ regulatory cells. J. Immunol. 167, 107–113 (2001).
Ciubotariu, R. et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J. Immunol. 161, 5193–5202 (1998).
Le Moine, A. & Goldman, M. Non-classical pathways of cell-mediated allograft rejection: new challenges for tolerance induction? Am. J. Transplant. 3, 101–106 (2003).
Seino, K. et al. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc. Natl. Acad. Sci. USA 98, 2577–2581 (2001).
Sonoda, K., Taniguchi, M. & Stein-Streilein, J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J. Immunol. 168, 2028–2034 (2002).
Yokoyama, W.M., Kim, S. & French, A.R. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22, 405–429 (2004).
Raulet, D.H. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat. Immunol. 5, 996–1002 (2004).
Lantz, O. & Bendelac, A. An invariant T cell receptor a chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4−8− T cells in mice and humans. J. Exp. Med. 180, 1097–1106 (1994).
Kronenberg, M. & Gapin, L. The unconventional lifestyle of NKT cells. Nat. Rev. Immunol. 2, 557–568 (2002).
Godfrey, D.I., MacDonald, R., Kronenberg, M., Smyth, M.J. & Van Kaer, L. NKT cells: what's in a name? Nat. Rev. Immunol. 4, 231–237 (2004).
Moretta, A. Natural killer cells and dendritic cells: rendezvous in abused tisues. Nat. Rev. Immunol. 2, 957–964 (2002).
Kondo, T. et al. Early increased chemokine expression and production in murine allogeneic skin grafts is mediated by natural killer cells. Transplantation 69, 969–977 (2000).
Sun, W. et al. IL-12p40-overexpressing immature dendritic cells induce T cell hyporesponsiveness in vitro but accelerate allograft rejection in vivo: role of NK cell activation and interferon-gamma production. Immunol. Lett. 94, 191–199 (2004).
Taniguchi, M., Harada, M., Kojo, S., Nakayama, T. & Wakao, H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu. Rev. Immunol. 21, 483–513 (2003).
Van Kaer, L. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol. Res. 30, 139–153 (2004).
Piccioli, D., Sbrana, S., Melandri, E. & Valiante, N.M. Contact-dependent stimulation and inibition of dendritic cells by natural killer cells. J. Exp. Med. 195, 335–341 (2002).
Ferlazzo, G. et al. The interaction between NK cells and dendritic cells in bacterial infections results in rapid induction of NK cell activation and in the lysis of uninfected dendritic cells. Eur. J. Immunol. 33, 306–313 (2003).
Hayakawa, Y. et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J. Immunol. 172, 123–129 (2004).
Pietra, G. et al. Comparative analysis of NK- or NK-CTL-mediated lysis of immature or mature autologous dendritic cells. Eur. J. Immunol. 33, 3427–3432 (2003).
Dowdell, K.C., Cua, D.J., Kirkman, E. & Stohlman, S.A. NK cells regulate CD4 responses prior to antigen encounter. J. Immunol. 171, 234–239 (2003).
Vankayalapati, R. et al. NK cells regulate CD8+ T cell effector function in response to an intracellular pathogen. J. Immunol. 172, 130–137 (2004).
Nicolls, M.R., Coulombe, M., Beilke, J., Gelhaus, H.C. & Gill, R.G. CD4-dependent generation of dominant transplantation tolerance induced by simultaneous perturbation of CD154 and LFA-1 pathways. J. Immunol. 169, 4831–4839 (2002).
Koller, B.H., Marrack, P., Kappler, J.W. & Smithies, O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science 248, 1227–1230 (1990).
Liao, N.S., Bix, M., Zijlstra, M., Jaenisch, R. & Raulet, D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science 253, 199–202 (1991).
Zhai, Y., Meng, L., Busuttil, R.W., Sayegh, M.H. & Kupiec-Weglinski, J.W. Activation of alloreactive CD8+ T cells operates via CD4-dependent and CD4-independent mechanisms and is CD154 blockade sensitive. J. Immunol. 170, 3024–3028 (2003).
Van Kaer, L., Ashton-Rickardt, P.G., Ploegh, H.L. & Tonegawa, S. TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell 71, 1205–1214 (1992).
Carbone, E. et al. A new mechanism of NK cell cytotoxicity activation: the CD40–CD40 ligand interaction. J. Exp. Med. 185, 2053–2060 (1997).
Ljunggren, H.G. & Karre, K. In search of the 'missing self': MHC molecules and NK cell reconition. Immunol. Today 11, 237–244 (1990).
Coudert, J.D., Coureau, C. & Guery, J.C. Preventing NK cell activation by donor dendritic cells enhances allospecific CD4 T cell priming and promotes Th type 2 responses to transplantation antigens. J. Immunol. 169, 2979–2987 (2002).
Sha, W.C. et al. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature 336, 73–76 (1988).
Bose, A., Inoue, Y., Kokko, K.E. & Lakkis, F.G. Cutting edge: perforin down-regulates CD4 and CD8 T cell-mediated immune responses to a transplanted organ. J. Immunol. 170, 1611–1614 (2003).
Rabinovich, B.A. et al. Activated, but not resting, T cells can be recognized and killed by syngeneic NK cells. J. Immunol. 170, 3572–3576 (2003).
Trambley, J. et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 104, 1715–1722 (1999).
Fort, M.M., Leach, M.W. & Rennick, D.M. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J. Immunol. 161, 3256–3261 (1998).
Maier, S. et al. Inhibition of natural killer cells results in acceptance of cardiac allografts in CD28−/− mice. Nat. Med. 7, 557–562 (2001).
Degli-Esposti, M.A. & Smyth, M.J. Close encounters of different kinds: dendritic cells and NK cells take centre stage. Nat. Rev. Immunol. 5, 112–124 (2005).
Mendiratta, S.K. et al. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6, 469–477 (1997).
Matsuda, J.L. et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl. Acad. Sci. USA 100, 8395–8400 (2003).
Acknowledgements
We would like to thank L. Gapin for experimental advice, T. Rubtsov for technical support and M. Sleater for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
NK and NKT cell activity increase in animals deficient in other class I pathways. (PDF 97 kb)
Supplementary Fig. 2
Anti-CD154 treatment permits NK cell killing and NKT cell IL-4 production. (PDF 83 kb)
Supplementary Table 1
CD8 T cells are not required for anti-CD154 induced allograft tolerance. (PDF 33 kb)
Rights and permissions
About this article
Cite this article
Beilke, J., Kuhl, N., Kaer, L. et al. NK cells promote islet allograft tolerance via a perforin-dependent mechanism. Nat Med 11, 1059–1065 (2005). https://doi.org/10.1038/nm1296
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1296
This article is cited by
-
Sterile inflammation in thoracic transplantation
Cellular and Molecular Life Sciences (2021)
-
Interactions between islets and regulatory immune cells in health and type 1 diabetes
Diabetologia (2021)
-
Host Expression of the CD8 Treg/NK Cell Restriction Element Qa-1 is Dispensable for Transplant Tolerance
Scientific Reports (2017)
-
A distinct innate lymphoid cell population regulates tumor-associated T cells
Nature Medicine (2017)
-
Diagnostic value of plasma and bronchoalveolar lavage samples in acute lung allograft rejection: differential cytology
Respiratory Research (2016)