Abstract
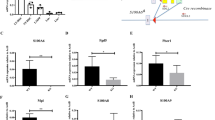
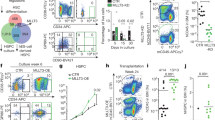
The molecular events that regulate engraftment and mobilization of hematopoietic stem cells and progenitors (HSC/Ps) are still incompletely defined1,2. We have examined the role of the Rho GTPases Rac1 and Rac2 in HSC engraftment and mobilization. Rac1, but not the hematopoietic-specific Rac2, is required for the engraftment phase of hematopoietic reconstitution, because Rac1−/− HSCs did not rescue in vivo hematopoiesis after transplantation, but deletion of Rac1 after engraftment did not impair steady-state hematopoiesis. Rac1−/− HSC/Ps showed impaired spatial localization to the endosteum but near-normal homing to the medullary cavity in vivo. Interaction with the bone marrow microenvironment in vitro was markedly altered. Whereas post-engraftment deletion of Rac1 alone did not impair hematopoiesis, deficiency of both Rac1 and Rac2 led to massive mobilization of HSCs from the marrow associated with ineffective hematopoiesis and intense selection for Rac-expressing HSCs. This mobilization was reversible by re-expression of Rac1. In addition, a rationally designed, reversible small-molecule inhibitor of Rac activation led to transient mobilization of engraftable HSC/Ps. Rac proteins thus differentially regulate engraftment and mobilization phenotypes, suggesting that these biological processes and steady-state hematopoiesis are biochemically separable and that Rac proteins may be important molecular targets for stem cell modification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lapidot, T., Dar, A. & Kollet, O. How do stem cells find their way home? Blood published online 12 May 2005 (10.1182/blood-2005-04-1417).
Papayannopoulou, T. Current mechanistic scenarios in hematopoietic stem/progenitor cell mobilization. Blood 103, 1580–1585 (2004).
Zhang, J. et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841 (2003).
Cottler-Fox, M.H. et al. Stem cell mobilization. Hematology (Am. Soc. Hematol. Educ. Program) 419–437 (2003).
Simmons, P.J., Levesque, J.P. & Zannettino, A.C. Adhesion molecules in haemopoiesis. Baillieres Clin. Haematol. 10, 485–505 (1997).
Levesque, J.P. & Simmons, P.J. Cytoskeleton and integrin-mediated adhesion signaling in human CD34+ hemopoietic progenitor cells. Exp. Hematol. 27, 579–586 (1999).
Lapidot, T. & Petit, I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30, 973–981 (2002).
Cancelas, J.A. et al. Peripheral blood CD34+ cell immunomagnetic selection in breast cancer patients: effect on hematopoietic progenitor content and hematologic recovery after high-dose chemotherapy and autotransplantation. Transfusion 38, 1063–1070 (1998).
Christopherson, K.W., Cooper, S., Hangoc, G. & Broxmeyer, H.E. CD26 is essential for normal G-CSF-induced progenitor cell mobilization as determined by CD26−/− mice. Exp. Hematol. 31, 1126–1134 (2003).
Gu, Y. et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302, 445–449 (2003).
Yang, F.C. et al. Rac and Cdc42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc. Natl Acad. Sci. USA 98, 5614–5618 (2001).
Didsbury, J., Weber, R.F., Bokoch, G.M., Evans, T. & Snyderman, R. rac, a novel ras-related family of proteins that are botulinum toxin substrates. J. Biol. Chem. 264, 16378–16382 (1989).
Haataja, L., Groffen, J. & Heisterkamp, N. Characterization of RAC3, a novel member of the Rho family. J. Biol. Chem. 272, 20384–20388 (1997).
Johnson, D.I. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54–105 (1999).
Yang, F.C. et al. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity 12, 557–568 (2000).
Gu, Y. et al. Biochemical and biological characterization of a human Rac2 GTPase mutant associated with phagocytic immunodeficiency. J. Biol. Chem. 276, 15929–15938 (2001).
Roberts, A.W. et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity 10, 183–196 (1999).
Filippi, M.D. et al. Localization of Rac2 via the C terminus and aspartic acid 150 specifies superoxide generation, actin polarity and chemotaxis in neutrophils. Nat. Immunol. 5, 744–751 (2004).
Papayannopoulou, T., Brice, M. & Stamatoyannopoulos, G. Hemoglobin F synthesis in vitro: evidence for control at the level of primitive erythroid stem cells. Proc. Natl Acad. Sci. USA 74, 2923–2927 (1977).
Peled, A. et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 (1999).
Driessen, R.L., Johnston, H.M. & Nilsson, S.K. Membrane-bound stem cell factor is a key regulator in the initial lodgment of stem cells within the endosteal marrow region. Exp. Hematol. 31, 1284–1291 (2003).
Jansen, M., Yang, F.C., Cancelas, J.A., Bailey, J.R. & Williams, D.A. Rac2-deficient hematopoietic stem cells show defective interaction with the hematopoietic microenvironment and long-term engraftment failure. Stem Cells 23, 335–346 (2005).
Scott, L.M., Priestley, G.V. & Papayannopoulou, T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol. Cell. Biol. 23, 9349–9360 (2003).
Gao, Y., Dickerson, J.B., Guo, F., Zheng, J. & Zheng, Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl Acad. Sci. USA 101, 7618–7623 (2004).
Roberts, A.W. et al. Genetic influences determining progenitor cell mobilization and leukocytosis induced by granulocyte colony-stimulating factor. Blood 89, 2736–2744 (1997).
Ploemacher, R.E., van der Sluijs, J.P., van Beurden, C.A., Baert, M.R. & Chan, P.L. Use of limiting-dilution type long-term marrow cultures in frequency analysis of marrow-repopulating and spleen colony-forming hematopoietic stem cells in the mouse. Blood 78, 2527–2533 (1991).
Gazitt, Y. Homing and mobilization of hematopoietic stem cells and hematopoietic cancer cells are mirror image processes, utilizing similar signaling pathways and occurring concurrently: circulating cancer cells constitute an ideal target for concurrent treatment with chemotherapy and antilineage-specific antibodies. Leukemia 18, 1–10 (2004).
Levesque, J.P., Hendy, J., Winkler, I.G., Takamatsu, Y. & Simmons, P.J. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp. Hematol. 31, 109–117 (2003).
Boggs, D.R. The total marrow mass of the mouse: a simplified method of measurement. Am. J. Hematol. 16, 277–286 (1984).
Nilsson, S.K., Johnston, H.M. & Coverdale, J.A. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97, 2293–2299 (2001).
Acknowledgements
This work was supported by US National Institutes of Health grant numbers R01 DK62752 (to D.A.W.) and R01 GM60523 (to Y.Z.). J.A.C. is a recipient of an award from the National Blood Foundation (United States). The authors want to thank M.-D. Filippi, H. Geiger and J. Mulloy for critical review of the manuscript; D. Witte for assistance in histologic analysis of organs; N. Krishnan, Y. Yamada, J. Bailey, V. Summey-Harner, C. Harris, F. Sadique, S. Homan and L. McMillan for technical assistance, and M. O'Leary, J. Hayden and E. Meunier for providing assistance in manuscript editing. We thank the Experimental Hematology Division Mouse and Flow Cores for services and Amgen, Inc. and Takara Bio for reagents.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have a pending patent on Rac inhibitors.
Supplementary information
Supplementary Fig. 1
Lineage reconstitution in peripheral blood of polyI:C treated mice (Rac-deficient) cells. (PDF 173 kb)
Supplementary Fig. 2
Content of HSC/P in BM and spleen of Rac-deficient mice. (PDF 267 kb)
Supplementary Fig. 3
Mobilization of HSC/P in Rac-deficient mice. (PDF 325 kb)
Rights and permissions
About this article
Cite this article
Cancelas, J., Lee, A., Prabhakar, R. et al. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med 11, 886–891 (2005). https://doi.org/10.1038/nm1274
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1274
This article is cited by
-
Validation of a small molecule inhibitor of PDE6D-RAS interaction with favorable anti-leukemic effects
Blood Cancer Journal (2022)
-
Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization
Leukemia (2019)
-
PTPσ inhibitors promote hematopoietic stem cell regeneration
Nature Communications (2019)
-
Mechanisms regulating mammalian spermatogenesis and fertility recovery following germ cell depletion
Cellular and Molecular Life Sciences (2019)
-
DOCK2 interacts with FLT3 and modulates the survival of FLT3-expressing leukemia cells
Leukemia (2017)