Abstract
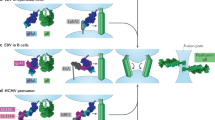
Human cytomegalovirus (HCMV) is a widespread opportunistic pathogen that causes birth defects in newborns and severe disease in immunocompromised individuals. The broad tropism of HCMV infection suggests that it uses multiple receptors. We recently showed that the epidermal growth factor receptor (EGFR) serves as a receptor for HCMV. Here we show that HCMV also uses integrin αvβ3 as a coreceptor. Upon infection, HCMV glycoproteins gB and gH independently bind to EGFR and αvβ3, respectively, to initiate viral entry and signaling. αvβ3 then translocates to lipid rafts where it interacts with EGFR to induce coordinated signaling. The coordination between EGFR and αvβ3 is essential for the early events of HCMV infection, including viral entry, RhoA downregulation, stress-fiber disassembly and viral nuclear trafficking. Our findings support a model in which EGFR and αvβ3 work together as coreceptors for HCMV entry and signaling. This discovery is fundamental to understanding HCMV pathogenesis and developing treatment strategies targeted to viral receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Huang, E.-S. & Kowalik, T.F. The pathogenicity of human cytomegalovirus: An overview. in Molecular Aspects of Human Cytomegalovirus Diseases (eds. Becker, Y., Darai, G. & Huang, E.S.) 1–45 (Springer, Berlin, 1993).
Speir, E. et al. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265, 391–394 (1994).
Mocarski, E.S. & Courcelle, C.T. Cytomegaloviruses and Their Replication. in Fields Virology Vol. 2 (eds. Knipe, D.M. & Howley, P.M.) 2629–2673 (Lippincott William & Wilkins, Philadelphia, 2001).
Macher, A.M. et al. Death in the AIDS patient: role of cytomegalovirus. N. Engl. J. Med. 309, 1454 (1983).
Shen, Y., Zhu, H. & Shenk, T. Human cytomagalovirus IE1 and IE2 proteins are mutagenic and mediate “hit-and-run” oncogenic transformation in cooperation with the adenovirus E1A proteins. Proc. Natl. Acad. Sci. USA 94, 3341–3345 (1997).
Lukac, D.M. & Alwine, J.C. Effects of human cytomegalovirus major immediate-early proteins in controlling the cell cycle and inhibiting apoptosis: studies with ts13 cells. J. Virol. 73, 2825–2831 (1999).
Cinatl, J., Scholz, M., Kotchetkov, R., Vogel, J.U. & Doerr, H.W. Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol. Med. 10, 19–23 (2004).
Flint, S.J., Enquist, L.W., Krug, M.R., Racaniello, V.R. & Skalka, A.M. Chapter 4 Virus attachment to host cell. in Principles of Virology: Molecular Biology, Pathogenesis, and Control. ch 4 101–131 (AWSM Press, Washington, D.C., 2000).
Feng, Y., Broder, C.C., Kennedy, P.E. & Berger, E.A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 272, 872–877 (1996).
Di Pasquale, G. et al. Identification of PDGFR as a receptor for AAV-5 transduction. Nat. Med. 9, 1306–1312 (2003).
Qing, K. et al. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat. Med. 5, 71–77 (1999).
Eppstein, D.A. et al. Epidermal growth factor receptor occupancy inhibits vaccinia virus infection. Nature 318, 663–665 (1985).
Terry-Allison, T. et al. HveA (herpesvirus entry mediator A), a coreceptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J. Virol. 72, 5802–5810 (1998).
Nemerow, G.R. & Cheresh, D.A. Herpesvirus hijacks an integrin. Nat. Cell Biol. 4, E69–E71 (2002).
Wang, X., Huong, S.M., Chiu, M.L., Raab-Traub, N. & Huang, E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424, 456–461 (2003).
Spear, P.G. & Longnecker, R. Herpesvirus entry: an update. J. Virol. 77, 10179–10185 (2003).
Fortunato, E.A., McElroy, A.K., Sanchez, I. & Spector, D.H. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 8, 111–119 (2000).
Johnson, R.A., Wang, X., Ma, X.L., Huong, S.M. & Huang, E.S. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75, 6022–6032 (2001).
Jones, N.L., Lewis, J.C. & Kilpatrick, B.A. Cytoskeletal disruption during human cytomegalovirus infection of human lung fibroblasts. Eur. J. Cell Biol. 41, 304–312 (1986).
Kalejta, R.F. & Shenk, T. Manipulation of the cell cycle by human cytomegalovirus. Front. Biosci. 7, d295–d306 (2002).
Schmidt, A. & Hall, M.N. Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14, 305–338 (1998).
Burridge, K. & Wennerberg, K. Rho and Rac take center stage. Cell 116, 167–179 (2004).
Schwartz, M.A. & Ginsberg, M.H. Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65–E68 (2002).
Hynes, R.O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 (1992).
Giancotti, F.G. & Tarone, G. Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19, 173–206 (2003).
Baron, W., Decker, L., Colognato, H. & ffrench-Constant, C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13, 151–155 (2003).
Anderson, R.G. The caveolae membrane system. Annu. Rev. Biochem. 67, 199–225 (1998).
Pike, L.J. Lipid rafts: bringing order to chaos. J. Lipid Res. 44, 655–667 (2003).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 (2000).
Pelkmans, L., Puntener, D. & Helenius, A. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296, 535–539 (2002).
Manes, S., del Real, G. & Martinez, A.C. Pathogens: raft hijackers. Nat. Rev. Immunol. 3, 557–568 (2003).
Li, E. et al. Association of p130CAS with phosphatidylinositol-3-OH kinase mediates adenovirus cell entry. J. Biol. Chem. 275, 14729–14735 (2000).
Wu, E. et al. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78, 3897–3905 (2004).
Ling, Y., Maile, L.A. & Clemmons, D.R. Tyrosine phosphorylation of the beta3-subunit of the alphaVbeta3 integrin is required for embrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol. Endocrinol. 17, 1824–1833 (2003).
Yurochko, A.D. et al. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-kappaB during infection. J. Virol. 71, 5051–5059 (1997).
Keay, S. & Baldwin, B. The human fibroblast receptor for gp86 of human cytomegalovirus is a phosphorylated glycoprotein. J. Virol. 66, 4834–4838 (1992).
Cudmore, S., Reckmann, I. & Way, M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 5, 142–148 (1997).
Ren, X.D., Kiosses, W.B. & Schwartz, M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Dimitrov, D.S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2, 109–122 (2004).
Schneider-Schaulies, J. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81, 1413–1429 (2000).
Smith, A.E. & Helenius, A. How viruses enter animal cells. Science 304, 237–242 (2004).
Fuller, A.O. & Perez-Romero, P. Mechanisms of DNA virus infection: entry and early events. Front. Biosci. 7, d390–d406 (2002).
Greber, U.F. Signalling in viral entry. Cell. Mol. Life Sci. 59, 608–626 (2002).
Moore, J.P. & Doms, R.W. The entry of entry inhibitors: a fusion of science and medicine. Proc. Natl. Acad. Sci. USA 100, 10598–10602 (2003).
Feire, A.L., Koss, H. & Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 101, 15470–15475 (2004).
Huang, E.S., Chen, S.T. & Pagano, J.S. Human cytomegalovirus. I. Purification and characterization of viral DNA. J. Virol. 12, 1473–1481 (1973).
Miller, N. & Hutt-Fletcher, L.M. A monoclonal antibody to glycoprotein gp85 inhibits fusion but not attachment of Epstein-Barr virus. J. Virol. 62, 2366–2372 (1988).
Kim, J.H., Saito, K. & Yokoyama, S. Chimeric receptor analyses of the interactions of the ectodomains of ErbB-1 with epidermal growth factor and of those of ErbB-4 with neuregulin. Eur. J. Biochem. 269, 2323–2329 (2002).
Tomlinson, C.C. & Damania, B. The K1 protein of Kaposi's sarcoma-associated herpesvirus activates the Akt signaling pathway. J. Virol. 78, 1918–1927 (2004).
Arreaza, G., Melkonian, K.A., LaFevre-Bernt, M. & Brown, D.A. Triton X-100-resistant membrane complexes from cultured kidney epithelial cells contain the Src family protein tyrosine kinase p62yes. J. Biol. Chem. 269, 19123–19127 (1994).
Acknowledgements
We are grateful to D. Clemmons for β3 and mutated β3 plasmids. We thank N. Raab-Traub, J. S. Pagano and D. Evers for discussion and critical review of this manuscript. This study was supported by Public Health Service research grants AI47468 from the National Institute of Allergy and Infectious Diseases and CA 19014 from the National Cancer Institute, US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
HCMV, but not HSV, induces transient interaction between EGFR and αvβ3. (PDF 26 kb)
Supplementary Fig. 2
RhoA signaling is not essential for HCMV entry. (PDF 29 kb)
Supplementary Fig. 3
A proposed model for the initiation of HCMV infection: receptor binding and signaling. (PDF 155 kb)
Supplementary Fig. 4
Integrin β1 can substitute for β3 in facilitating HCMV infection. (PDF 30 kb)
Supplementary Fig. 5
Analysis of the purified HCMV virions by SDS-polyacrylaide gel electrophoresis and electron microscopy. (PDF 56 kb)
Rights and permissions
About this article
Cite this article
Wang, X., Huang, D., Huong, SM. et al. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat Med 11, 515–521 (2005). https://doi.org/10.1038/nm1236
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1236
This article is cited by
-
Modulation of host cell signaling during cytomegalovirus latency and reactivation
Virology Journal (2021)
-
Differential Expression of PDGF Receptor-α in Human Placental Trophoblasts Leads to Different Entry Pathways by Human Cytomegalovirus Strains
Scientific Reports (2020)
-
Viral journeys on the intracellular highways
Cellular and Molecular Life Sciences (2018)
-
A comparative review of viral entry and attachment during large and giant dsDNA virus infections
Archives of Virology (2017)
-
Effect of equine herpesvirus type 1 (EHV-1) infection of nasal mucosa epithelial cells on integrin alpha 6 and on different components of the basement membrane
Archives of Virology (2016)