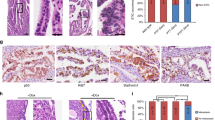
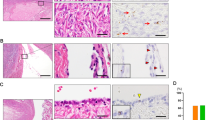
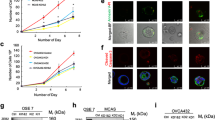
Abstract
Although epithelial ovarian cancers (EOCs) have been thought to arise from the simple epithelium lining the ovarian surface or inclusion cysts, the major subtypes of EOCs show morphologic features that resemble those of the müllerian duct–derived epithelia of the reproductive tract. We found that HOX genes, which normally regulate müllerian duct differentiation, are not expressed in normal ovarian surface epithelium (OSE), but are expressed in different EOC subtypes according to the pattern of müllerian-like differentiation of these cancers. Ectopic expression of Hoxa9 in tumorigenic mouse OSE cells gave rise to papillary tumors resembling serous EOCs. In contrast, Hoxa10 and Hoxa11 induced morphogenesis of endometrioid-like and mucinous-like EOCs, respectively. Hoxa7 showed no lineage specificity, but promoted the abilities of Hoxa9, Hoxa10 and Hoxa11 to induce differentiation along their respective pathways. Therefore, inappropriate activation of a molecular program that controls patterning of the reproductive tract could explain the morphologic heterogeneity of EOCs and their assumption of müllerian-like features.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Feeley, K.M. & Wells, M. Precursor lesions of ovarian epithelial malignancy. Histopathology 38, 87–95 (2001).
Auersperg, N., Wong, A.S., Choi, K.C., Kang, S.K. & Leung, P.C. Ovarian surface epithelium: Biology, endocrinology, and pathology. Endocr. Rev. 22, 255–288 (2001).
Shih, I. & Kurman, R.J. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 164, 1511–1518 (2004).
Mills, G.B. et al. The role of genetic abnormalities of PTEN and the phosphatidylinositol 3-kinase pathway in breast and ovarian tumorigenesis, prognosis, and therapy. Semin. Oncol. 28, 125–141 (2001).
Enomoto, T., Weghorst, C.M., Inoue, M., Tanizawa, O. & Rice, J.M. K-ras activation occurs frequently in mucinous adenocarcinomas and rarely in other common epithelial tumors of the human ovary. Am J Pathol. 139, 777–785 (1991).
Dinulescu, D.M. et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat. Med. 11, 63–70 (2005).
Orsulic, S. et al. Induction of ovarian cancer by defined multiple genetic changes in a mouse model system. Cancer Cell 1, 53–62 (2002).
Connolly, D.C. et al. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res. 63, 1389–1397 (2003).
Fleshken-Nikitin, A., Choi, K., Eng, J.P., Shmidt, E.N. & Nikitin, A.Y. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 63, 3459–3463 (2003).
McGinnis, W., Levine, M.S., Hafen, E., Kuroiwa, A. & Gehring, W.J. A conserved DNA sequence in homeotic genes of the Drosophila Antennapedia and bithorax complexes. Nature 308, 428–433 (1984).
Hsieh-Li, H.M. et al. Hoxa-11 structure, extensive antisense transcription and function in male and female sterility. Development 121, 1373–1385 (1995).
Benson, G.V. et al. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development 122, 2687–2696 (1996).
Taylor, H.S., Vanden Heuvel, G.B. & Igarashi, P. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol. Reprod. 57, 1338–1345 (1997).
Roby, K.F. et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis 21, 585–591 (2000).
Schwartz, D.R. et al. Gene expression in ovarian cancer reflects both morphology and biological behavior, distinguishing clear cell from other poor-prognosis ovarian carcinomas. Cancer Res. 62, 4722–4729 (2002).
Lu, K.H. et al. Selection of potential markers for epithelial ovarian cancer with gene expression arrays and recursive descent partition analysis. Clin. Cancer Res. 10, 3291–3300 (2004).
Drapkin, R., Crum, C.P. & Hecht, J.L. Expression of candidate tumor markers in ovarian carcinoma and benign ovary: evidence for a link between epithelial phenotype and neoplasia. Hum. Pathol. 35, 1014–1021 (2004).
Hough, C.D. et al. Large-scale serial analysis of gene expression reveals genes differentially expressed in ovarian cancer. Cancer Res. 60, 6281–6287 (2000).
Bradgate, M.G. et al. Binding of anti-EMA, AGF 4:48 and the lectin UEA-1 to human ovarian carcinomas: histological and clinical correlations. Br. J. Obstet. Gynecol. 96, 854–860 (1989).
Albarracin, C.T., Jafri, J., Montag, A.G., Hart, J. & Kuan, S.F. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum. Pathol. 31, 672–677 (2000).
Ordonez, N.G. Application of mesothelin immunostaining in tumor diagnosis. Am. J. Surg. Pathol. 27, 1418–1428 (2003).
Naora, H., Montz, F.J., Chai, C.Y. & Roden, R.B.S. Aberrant expression of homeobox gene HOXA7 is associated with müllerian-like differentiation of epithelial ovarian tumors and the generation of a specific autologous antibody response. Proc. Natl. Acad. Sci. USA 98, 15209–15214 (2001).
Korkolopoulou, P. et al. The combined evaluation of p27Kip1 and Ki-67 expression provides independent information on overall survival of ovarian carcinoma patients. Gynecol. Oncol. 85, 404–414 (2002).
Naora, H. et al. A serologically identified tumor antigen encoded by a homeobox gene promotes growth of ovarian epithelial cells. Proc. Natl. Acad. Sci. USA 98, 4060–4065 (2001).
Care, A. et al. Enforced expression of HOXB7 promotes hematopoietic stem cell proliferation and myeloid-restricted progenitor differentiation. Oncogene 18, 1993–2001 (1999).
Liu, J. et al. A genetically defined model for human ovarian cancer. Cancer Res. 64, 1655–1663 (2004).
Dubeau, L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: Does the emperor have no clothes? Gynecol. Oncol. 72, 437–442 (1999).
Magli, M.C., Largman, C. & Lawrence, H.J. Effects of HOX homeobox genes in blood cell differentiation. J. Cell. Physiol. 173, 168–177 (1997).
Ma, L., Benson, G.V., Lim, H., Dey, S.K. & Maas, R.L. AbdominalB (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in müllerian duct by the synthetic estrogen diethylstillbestrol (DES). Dev. Biol. 197, 141–154 (1998).
Sato, N. et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid and clear cell carcinoma of the ovary. Cancer Res. 60, 7052–7056 (2000).
Capo-chichi, C.D. et al. Anomalous expression of epithelial differentiation-determining GATA factors in ovarian tumorigenesis. Cancer Res. 63, 4967–4977 (2003).
Kroon, E. et al. Hoxa9 transforms primary bone marrow cells through specific collaboration with Meis1a but not Pbx1b. EMBO J. 17, 3714–3725 (1998).
Lim, H., Ma, L., Ma, W.G., Maas, R.L. & Dey, S.K. Hoxa10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol. Endocrinol. 13, 1005–1017 (1999).
Kobayashi, A. & Behringer, R.R. Developmental genetics of the female reproductive tract in mammals. Nat. Rev. Genet. 4, 969–980 (2003).
He, W.W. et al. A novel human prostate-specific, androgen-regulated homeobox gene (NKX3.1) that maps to 8p21, a region frequently deleted in prostate cancer. Genomics 43, 69–77 (1997).
Kim, M.J. et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc. Natl. Acad. Sci. USA 99, 2884–2889 (2002).
Bonhomme, C. et al. The Cdx2 homeobox gene has a tumor suppressor function in the distal colon in addition to a homeotic role during gut development. Gut 52, 1465–1471 (2003).
Abate-Shen, C. Deregulated homeobox gene expression in cancer: cause or consequence? Nature Rev. Cancer 2, 777–785 (2002).
Ford, H.L., Kabingu, E.N., Bump, E.A., Mutter, G.L. & Pardee, A.B. Abrogation of the G2 cell cycle checkpoint associated with overexpression of HSIX1: a possible mechanism of breast carcinogenesis. Proc. Natl. Acad. Sci. USA 95, 12608–12613 (1998).
Thorsteinsdottir, U. et al. Overexpression of HOXA10 in murine hematopoietic cells perturbs both myeloid and lymphoid differentiation and leads to acute myeloid leukemia. Mol. Cell. Biol. 17, 495–505 (1997).
Acknowledgements
We thank J. Hung and K. Head for technical assistance, R. Broaddus for providing additional tissue specimens, G. Mills, Y. Lu, R. Bast and R. Behringer (University of Texas M.D. Anderson Cancer Center) for discussions, and N. Auersperg (University of British Columbia) for IOSE-29 cells. This work was supported by US Department of the Army Award DAMD 17-03-1-0219 (H.N.); by US National Institutes of Health/National Cancer Institute Grants R21 CA102108 (H.N), R01 CA101826 (H.N.), P01 CA64602 (J.L.); by a University of Texas M.D. Anderson Cancer Center Institutional Research Grant (H.N.); by an award from Cancer Fighters of Houston (H.N.); by American Cancer Society Grant RSG-04-028-1-CCE (J.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
RT-PCR analysis of HOX expression in normal reproductive tract tissues and in EOCs. (PDF 103 kb)
Supplementary Fig. 2
Specificity of HOX antibodies determined by siRNA knockdown. (PDF 332 kb)
Supplementary Fig. 3
Ectopic HOX expression in stably transfected MOSEC cell lines. (PDF 143 kb)
Supplementary Fig. 4
HOX expression in tumors dervied from stably transfected MOSEC cells. (PDF 399 kb)
Supplementary Fig. 5
Morphologic alterations induced by HOXA7 in SKOV3 cells. (PDF 144 kb)
Rights and permissions
About this article
Cite this article
Cheng, W., Liu, J., Yoshida, H. et al. Lineage infidelity of epithelial ovarian cancers is controlled by HOX genes that specify regional identity in the reproductive tract. Nat Med 11, 531–537 (2005). https://doi.org/10.1038/nm1230
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1230
This article is cited by
-
Successful in vitro fertilization following conservative surgery for synchronous endometrioid tumor of ovary and uterus
Journal of Ovarian Research (2023)
-
The DNA methylome of cervical cells can predict the presence of ovarian cancer
Nature Communications (2022)
-
ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer
Journal of Experimental & Clinical Cancer Research (2021)
-
Neoadjuvant metformin added to conventional chemotherapy synergizes anti-proliferative effects in ovarian cancer
Journal of Ovarian Research (2020)
-
Non-coding somatic mutations converge on the PAX8 pathway in ovarian cancer
Nature Communications (2020)