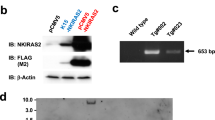
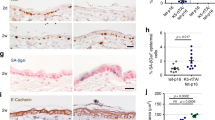
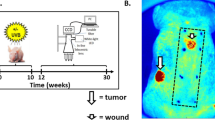
Abstract
Basal cell carcinomas, the commonest human skin cancers, consistently have abnormalities of the hedgehog signaling pathway and often have PTCH gene mutations. We report here that Ptch+/– mice develop primordial follicular neoplasms resembling human trichoblastomas, and that exposure to ultraviolet radiation or ionizing radiation results in an increase in the number and size of these tumors and a shift in their histologic features so that they more closely resemble human basal cell carcinoma. The mouse basal cell carcinomas and trichoblastoma-like tumors resemble human basal cell carcinomas in their loss of normal hemidesmosomal components, presence of p53 mutations, frequent loss of the normal remaining Ptch allele, and activation of hedgehog target gene transcription. The Ptch mutant mice provide the first mouse model, to our knowledge, of ultraviolet and ionizing radiation-induced basal cell carcinoma-like tumors, and also demonstrate that Ptch inactivation and hedgehog target gene activation are essential for basal cell carcinoma tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Miller, D.L. & Weinstock, M.A. Non-melanoma skin cancer in the United States: incidence. J. Am. Acad. Dermatol. 30, 774–778 (1994).
Hahn, H. et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell 85, 841–851 (1996).
Johnson, R.L. et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science 272, 1668–1671 (1996).
Vorechovsky, I., Unden, A.B., Sandstedt B., Toftgard, R. & Stahle-Backdahl, M. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 57, 4677–4681 (1997).
Raffel, C. et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 57, 842–845 (1997).
Xie, J. et al. Mutations of the PATCHED gene in several types of sporadic extracutaneous tumors. Cancer Res 57, 2369–2372, (1997).
Gailani, M.R. et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nature Genet. 14, 78–81 (1996).
Kallassy, M. et al. Patched (ptch)-associated preferential expression of smoothened (smoh) in human basal cell carcinoma of the skin. Cancer Res. 57, 4731–4735 (1997).
Dahmane, N., Lee, J., Robins, P., Heller, P. & Ruiz i Altaba, A. Activation of the transcription factor Gli1 and the sonic hedgehog signalling pathway in skin tumours. Nature 389, 876–880 (1997).
Green, J., Leigh, I.M., Poulsom R., Quinn, A.G. Basal cell carcinoma development is associated with induction of the expression of the transcription factor Gli-1. Br. J. Dermatol. 139, 911–915 (1998).
Bogovski, P. in Tumours of the Mouse. Pathology of Tumours in Laboratory Animals Vol. 2 (eds. Turusov, V.S. & Mohr, U.) 11–12 (International Agency for Research on Cancer, Lyon, 1994).
Oro, A.E. et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276, 817–821 (1997).
Xie, J. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998).
Goodrich, L.V., Milenkovic, L., Higgins, K.M., & Scott, M.P. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science 277, 1109–1113 (1997).
Hahn, H. et al. Rhabdomyosarcomas and radiation hypersensitivity in a mouse model of Gorlin syndrome. Nature Med. 4, 619–622 (1998).
Walsh, N., Ackerman, A.B. Infundibulocystic basal cell carcinoma: a newly described variant. Mod. Path. 3, 599–608 (1990).
Gorlin, R.J. Nevoid basal cell carcinoma syndrome. Medicine 66, 98–109 (1987).
Hashimoto, K., Howell, J.B., Yamanishi, Y., Holubar, K. & Bernhard, R. Electron microscopic studies of palmar and plantar pits of nevoid basal cell epithelioma. J. Invest. Dermatol. 59, 380–393 (1972).
Karagas, M.R. et al. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. J. Natl. Cancer Inst. 88, 1848–1852 (1996).
O'Malley, S. et al. Multiple neoplasms following craniospinal irradiation for medulloblastoma in a patient with nevoid basal cell carcinoma syndrome. J. Neurosurg. 86, 286–288 (1997).
Miller, S.J., Tung-Tien, S. & Lavker, R.M. Hair follicles, stem cells, and skin cancer. J. Invest. Dermatol. 100, 288S–294S (1993).
Markey, A.C., Lane, E.B., MacDonald, D.M. & Leigh, I.M. Keratin expression in basal cell carcinomas. Br. J. Dermatol. 126, 154–160 (1992).
Fairley, J.A., Heintz, P.W., Neuburg, M., Diaz, L.A. & Giudice, G.J. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br. J. Dermatol. 133, 385–391 (1995).
Bahadoran, P. et al. Altered expression of the hemidesmosome-anchoring filament complex proteins in basal cell carcinoma: possible role in the origin of peritumoral lacunae. Br. J. Dermatol. 136, 35–42 (1997).
Smoller, B.R., Van de Rijn, M., Lebrun, D. & Warnke, R.A. Bcl-2 expression reliably distinguishes trichoepitheliomas from basal cell carcinomas. Br. J. Dermatol. 131, 28–31 (1994).
Bonifas, J.M., Bare, J.W., Kerschmann, R.L., Master, S.P. & Epstein, E.H. Jr. Parental origin of chromosome 9q22.3-q31 lost in basal cell carcinomas from basal cell nevus syndrome patients. Hum. Mol. Genet. 3, 447–448 (1994).
Van der Riet, P. et al. Progression of basal cell carcinoma through loss of chromosome 9 and inactivation of a single p53 allele. Cancer Res. 54, 25–27 (1994).
Gailani, M.R. et al. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J. Natl. Cancer Inst. 88, 349–354 (1996).
Grando, S.A. et al. Nodular basal cell carcinoma in vivo vs. in vitro. Arch. Dermatol. 132, 1185–1193 (1996).
Nishigori, C., Yarosh, D.B., Danawho, C. & Kripke, M.L. The immune system in ultraviolet carcinogenesis. J. Invest. Dermatol. Symp. Proc. 1, 143–146 (1996).
Della Porta, G., Terracini, B., Dammert, K. & Shubik, P. Histopathology of tumors induced in mice treated with polyoxyethylene sorbitan monostearate. J. Natl. Cancer Inst. 25, 573–605 (1960).
Quinn, A.G., Campbell, C., Healy, E. & Rees, J.L. Chromosome 9 allele loss occurs in both basal and squamous cell carcinomas of the skin. J. Invest. Dermatol. 102, 300–303 (1994).
Epstein, J.H. Comparison of the carcinogenic and cocarcinogenic effects of ultraviolet light on hairless mice. J. Natl. Cancer Inst. 34, 741–745 (1965).
Epstein, J.H. in Models in Dermatology Vol 2 (eds. Maibach, H. & Lowe, N.J.) 303–312 (Karger, Basel, 1985).
Guo, L., Yu, Q.-C. & Fuchs, E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 12, 973–986 (1993).
Liu, Z. et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J. Clin. Invest. 92, 2480–2488 (1993).
Aberdam, D. et al. Developmental expression of nicein adhesion protein (laminin 5) subunits suggests multiple morphogenic roles. Cell Adhes. and Commun. 2, 115–129 (1994).
Krajewski, S. et al. Immunohistochemical determination of in vivo distribution of bax, a dominant inhibitor of bcl-2. Am. J. Path. 145, 1323–1336 (1994).
Kress, S. et al. Carcinogen-specific mutational pattern in the p53 gene in ultraviolet B radiation-induced squamous cell carcinomas of mouse skin. Cancer Res. 52, 6400–6403 (1992).
Garrigue, J.L. et al. Optimization of the mouse ear swelling test for in vivo and in vitro studies of weak contact sensitizers. Contact Dermatitis 30, 231–237 (1994).
Acknowledgements
We thank L. Goodrich for providing the Ptch +/– mouse, M. Weinstein and S. Pennypacker for technical assistance, and R. Szabo for his critical input. This work was supported by grants from the National Institutes of Health (AR39959, AR4311 and CA81888) (E.H.E.), fellowships from the Dermatology Foundation (M.A.), and donations from P. Hughes and from the Michael J. Rainen Family Foundation. M.P.S. is an Investigator of the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Aszterbaum, M., Epstein, J., Oro, A. et al. Ultraviolet and ionizing radiation enhance the growth of BCCs and trichoblastomas in patched heterozygous knockout mice. Nat Med 5, 1285–1291 (1999). https://doi.org/10.1038/15242
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/15242
This article is cited by
-
Human Skin Cancer: an Overview Of Animal, Ex Vivo, and In Vitro Models
Current Dermatology Reports (2022)
-
Patched1 haploinsufficiency severely impacts intermediary metabolism in the skin of Ptch1+/−/ODC transgenic mice
Scientific Reports (2019)
-
Characterisation of resistance mechanisms developed by basal cell carcinoma cells in response to repeated cycles of Photodynamic Therapy
Scientific Reports (2019)
-
Bone marrow-derived mesenchymal stromal cells promote colorectal cancer cell death under low-dose irradiation
British Journal of Cancer (2018)
-
Hedgehog pathway mutations drive oncogenic transformation in high-risk T-cell acute lymphoblastic leukemia
Leukemia (2018)