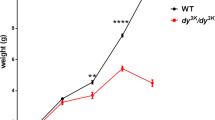
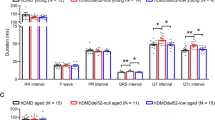
Abstract
Dystrophin-deficient mice (mdx) expressing a truncated (trc) utrophin transgene show amelioration of the dystrophic phenotype. Here we report a multifunctional study demonstrating that trc-utrophin expression leads to major improvements of the mechanical performance of muscle (that is, force development, mechanical resistance to forced lengthenings and maximal spontaneous activity) and of the maintenance of the intracellular calcium homeostasis. These are two essential functions of muscle fibers, known to be impaired in mdx mouse muscles and Duchenne muscular dystrophy (DMD) patients. Our results bring strong support to the hypothesis that muscle wasting in dystrophin-deficient DMD patients could be prevented by upregulation of utrophin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Blake D.J., Tinsley J.M. & Davies K.E. Utrophin: a structural and functional comparison to dystrophin. Brain Pathol. 6, 37–47 (1996).
Tinsley, J.M. et al. Primary structure of dystrophin-related protein. Nature 360, 591–592 (1992).
Pons, F. et al. A homologue of dystrophin is expressed at the neuromuscular junctions of normal individuals and DMD patients, and of normal and mdx mice: Immunological evidence. FEBS Lett. 282, 161–165 (1991).
Matsumura, K., Ervasti, J., Ohlendieck, K., Kahl, S. & Campbell, K. Association of dystrophin-related protein with dystrophin associated proteins in mdx mouse muscle. Nature 360, 588–591 (1993).
Mizumo, Y., Nonaka, I., Hirai, S. & Ozawa, E. Reciprocal expression of dystrophin and utrophin in muscles of Duchenne muscular dystrophy patients, female DMD carriers and control subjects. J. Neurol. Sci. 119, 43–52 (1993).
Karpati, G. et al. Localization and quantitation of the chromosome 6-encoded dystrophin-related protein in normal and pathological human muscle. J. Neuropathol. Exp. Neural. 52, 119–128 (1993).
Tinsley, J.M. & Davies, K.E. Utrophin: A potential replacement for dystrophin? Neuromusc. Disord. 3, 537–539 (1993).
Ragot Th. et al. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature 361, 647–650 (1993).
Phelps, S.F. et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum. Mol. Genet. 4, 1251–1258 (1995).
Deconinck N., Ragot T., Maréchal, G., Pérricaudet M. & Gillis J.M. Functional protection of dystrophic mouse (mdx) muscles after adenovirus-mediated transfer of a dystrophin minigene. Proc. Nat. Acad. Sci. USA 93, 3570–3574 (1996).
Tinsley, J.M. et al. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature 384, 349–353 (1996).
Karpati, G. Utrophin muscles in on the action. Nature Med. 3, 22–23 (1997).
Coulton, G.R., Curtin N.A., Morgan J.E. & Partridge T.A. The mdx mouse skeletal muscle myopathy. II. Contractile properties. Neuropathol. Appl. Neurobiol. 14, 299–314 (1988).
Stedman, H.H. et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352, 536–539 (1991).
Weller B., Karpati G. & Carpenter S. Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J. Neurol. Sci. 100, 9–13 (1990).
Moens, P., Baatsen, P.H.W.W. & Maréchal, G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J. Musc. Res. Cell Motil. 14, 446–451 (1993).
Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M. & Sweeney H.L. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA 90, 3710–3714 (1993).
Carlson, C.G. & Makiejus, R.V. A non-invasive procedure to detect muscle weakness in the mdx mouse. Muscle Nerve 13, 480–484 (1990).
Bodensteiner, J.B. & Engel, A.G., Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: A study of 567,000 muscle fibres in 114 biopsies. Neurology 28, 439–446 (1978).
Gailly Ph Boland B., Himpens B., Casteels R. & Gillis, J.M. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium 14, 473–483 (1993).
Head, S.I. Membrane potential, resting calcium and calcium transients in isolated muscle fibres from normal and dystrophic mice. J. Physiol. 469, 11–19 (1993).
Pressmar, J., Brinkmeier, H., Seewald, M.J., Naumann, T. & Rüdel, R., Cellular Ca2+ concentrations are not elevated in resting cultured muscle from Duchenne (DMD) patients and in MDX mouse muscle fibres Pfluegers Arch. 426, 499–505 (1994).
Turner P.R., Fong P., Denetclaw W.F. & Steinhardt, R.A. Increased calcium influx in dystrophic muscle. J. Cell Biol. 115, 1701–1712 (1991).
Leijendekker, W.J., Passaquin A.C., Metzinger L. & Ruegg, U.T. Regulation of cytosolic calcium in skeletal muscle cells of the mdx mouse under conditions of stress. Br. J. Pharmacol. 118, 611–616 (1996).
Grynkiewicz, G., Poenie, M. & Tsien, R.Y. A new generation of Ca2+ indicators with greatly improved fluorescent properties. J. Biol. Chem. 260, 3440–50 (1985).
Sunada, Y. & Campbell, K. Dystrophin-glycoprotein complex: Molecular organization and critical roles in skeletal muscle. Curr. Opin Neurol. 8, 379–384 (1995).
Takeshima, Y. et al. Amino-terminal deletion of 53% of dystrophin results in an intermediate Duchenne-Becker muscular dystrophy phenotype. Neurology 44, 1648–1651 (1994).
Winder, S.J. & Kendrick-Jones, J. Calcium/calmodulin-dependent regulation of the NH, terminal F-actin binding domain of utrophin. FEBS Lett. 357, 125–128 (1995).
Sugita, H., Takemitsu, M., Koga, R., Ishiura, S. & Arahata, K. The expression of utrophin in mdx mouse muscle dystrophy. Acta Cardiomiol. 5, 11–16 (1993).
Haws, C.M. & Lansman, J.B. Developmental regulation of mechanosensitive calcium channels in skeletal muscle from normal and mdx mice. Proc. R. Soc. Land. B 245, 173–177 (1991).
McArdle, A., Edwards, R.H.T. & Jackson M.J. Time course of changes in plasma membrane permeability in the dystrophin-deficient mdx mouse. Muscle Nerve 17, 1378–1384 (1994).
Menke, A. & Jockusch, H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature 349, 69–71 (1991).
Webster, C., Silberstein L. Hays A.P. & Blau H.M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52, 502–13 (1988).
Bailey, N.T. Statistical Methods in Biology (English Univ. Press, London, 1959).
Cox, G.A. et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature 364, 725–729 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Deconinck, N., Tinsley, J., Backer, F. et al. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med 3, 1216–1221 (1997). https://doi.org/10.1038/nm1197-1216
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1197-1216
This article is cited by
-
Biomarkers for Duchenne muscular dystrophy progression: impact of age in the mdx tongue spared muscle
Skeletal Muscle (2023)
-
Endogenous bioluminescent reporters reveal a sustained increase in utrophin gene expression upon EZH2 and ERK1/2 inhibition
Communications Biology (2023)
-
Lifetime analysis of mdx skeletal muscle reveals a progressive pathology that leads to myofiber loss
Scientific Reports (2020)
-
High-throughput identification of post-transcriptional utrophin up-regulators for Duchenne muscle dystrophy (DMD) therapy
Scientific Reports (2020)
-
Humanizing the mdx mouse model of DMD: the long and the short of it
npj Regenerative Medicine (2018)