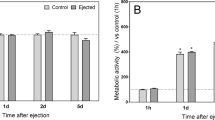
Abstract
Structural allograft healing is limited because of a lack of vascularization and remodeling. To study this we developed a mouse model that recapitulates the clinical aspects of live autograft and processed allograft healing. Gene expression analyses showed that there is a substantial decrease in the genes encoding RANKL and VEGF during allograft healing. Loss-of-function studies showed that both factors are required for autograft healing. To determine whether addition of these signals could stimulate allograft vascularization and remodeling, we developed a new approach in which rAAV can be freeze-dried onto the cortical surface without losing infectivity. We show that combination rAAV-RANKL- and rAAV-VEGF-coated allografts show marked remodeling and vascularization, which leads to a new bone collar around the graft. In conclusion, we find that RANKL and VEGF are necessary and sufficient for efficient autograft remodeling and can be transferred using rAAV to revitalize structural allografts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garbuz, D.S., Masri, B.A. & Czitrom, A.A. Biology of allografting. Orthop. Clin. North. Am. 29, 199–204 (1998).
Goldberg, V.M. & Stevenson, S. The biology of bone grafts. Semin. Arthroplasty 4, 58–63 (1993).
Einhorn, T.A. The cell and molecular biology of fracture healing. Clin. Orthop. S7–S21 (1998).
Burchardt, H. Biology of bone transplantation. Orthop. Clin. North. Am. 18, 187–196 (1987).
Gould, S.E., Rhee, J.M., Tay, B.-B., Otsuka, N.Y. & Bradford, D.S. Cellular contribution of bone graft to fusion. J. Orthop. Res. 18, 920–927 (2000).
Enneking, W.F. & Campanacci, D.A. Retrieved human allografts: a clinicopathological study. J. Bone Joint Surg. Am. 83-A, 971–986 (2001).
Lord, C.F., Gebhardt, M.C., Tomford, W.W. & Mankin, H.J. Infection in bone allografts. Incidence, nature, and treatment. J. Bone Joint Surg. Am. 70, 369–376 (1988).
Berrey, B.H., Jr., Lord, C.F., Gebhardt, M.C. & Mankin, H.J. Fractures of allografts. Frequency, treatment, and end-results. J. Bone Joint Surg. Am. 72, 825–833 (1990).
Colnot, C., Thompson, Z., Miclau, T., Werb, Z. & Helms, J.A. Altered fracture repair in the absence of MMP9. Development 130, 4123–4133 (2003).
Kon, T. et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 16, 1004–1014. (2001).
Ferrara, N., Gerber, H.P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Boyle, W.J., Simonet, W.S. & Lacey, D.L. Osteoclast differentiation and activation. Nature 423, 337–342. (2003).
Tiyapatanaputi, P. et al. A novel murine segmental femoral graft model. J Orthop Res 22, 1254–1260 (2004).
Hadjiargyrou, M. et al. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J. Biol. Chem. 277, 30177–30182 (2002).
Zhang, X. et al. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J. Clin. Invest. 109, 1405–1415 (2002).
Bonadio, J., Smiley, E., Patil, P. & Goldstein, S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat. Med. 5, 753–759 (1999).
Schwarz, E.M. The adeno-associated virus vector for orthopaedic gene therapy. Clin. Ortho. & Rel. Res. 379S, S31–S39 (2000).
Rabinowitz, J.E. & Samulski, R.J. Building a better vector: the manipulation of AAV virions. Virology 278, 301–308 (2000).
Wu, D., Razzano, P. & Grande, D.A. Gene therapy and tissue engineering in repair of the musculoskeletal system. J. Cell. Biochem. 88, 467–481 (2003).
Gamradt, S.C. & Lieberman, J.R. Genetic modification of stem cells to enhance bone repair. Ann. Biomed. Eng. 32, 136–147 (2004).
Boden, S.D., Kang, J., Sandhu, H. & Heller, J.G. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine 27, 2662–2273 (2002).
Friedlaender, G.E. OP-1 clinical studies. J. Bone Joint Surg. Am. 83-A Suppl 1, S160–S161 (2001).
Lieberman, J.R., Ghivizzani, S.C. & Evans, C.H. Gene transfer approaches to the healing of bone and cartilage. Mol. Ther. 6, 141–147 (2002).
Baltzer, A.W. & Lieberman, J.R. Regional gene therapy to enhance bone repair. Gene Ther. 11, 344–350 (2004).
Sandhu, H.S., Boden, S.D., An, H., Kang, J. & Weinstein, J. BMPs and gene therapy for spinal fusion: summary statement. Spine 28, S85 (2003).
Musgrave, D.S. et al. Ex vivo gene therapy to produce bone using different cell types. Clin. Orthop. 378, 290–305 (2000).
Hidaka, C. et al. Acceleration of cartilage repair by genetically modified chondrocytes over expressing bone morphogenetic protein-7. J. Orthop. Res. 21, 573–583 (2003).
Verma, I.M. & Somia, N. Gene therapy -- promises, problems and prospects. Nature 389, 239–242 (1997).
Bos, G.D., Goldberg, V.M., Powell, A.E., Heiple, K.G. & Zika, J.M. The effect of histocompatibility matching on canine frozen bone allografts. J. Bone Joint Surg. Am. 65, 89–96 (1983).
Stevenson, S., Li, X.Q., Davy, D.T., Klein, L. & Goldberg, V.M. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. Quantification of a complex process and structure. J. Bone Joint Surg. Am. 79, 1–16 (1997).
Childs, L.M. et al. In vivo RANK signaling blockade using the receptor activator of NF- kappaB:Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J. Bone Miner Res. 17, 192–199 (2002).
Childs, L.M., Goater, J.J., O'Keefe, R.J. & Schwarz, E.M. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris- induced osteolysis. J. Bone Joint Surg. Am. 83-A, 1789–1797. (2001).
Musatov, S. et al. Inhibition of neuronal phenotype by PTEN in PC12 cells. Proc. Natl. Acad. Sci. USA 101, 3627–3631 (2004).
Goater, J. et al. Empirical advantages of adeno associated viral vectors in vivo gene therapy for arthritis. J. Rheumatol. 27, 983–989 (2000).
Ulrich-Vinther, M. et al. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J. Bone Joint Surg. Am. 84-A, 1405–1412 (2002).
Zhang, R. et al. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J. Biol. Chem. 278, 51267–51276 (2003).
Lacey, D.L. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 (1998).
Peng, H. et al. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J. Clin. Invest. 110, 751–759 (2002).
Xiao, X., Li, J. & Samulski, R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72, 2224–2232 (1998).
Deckers, M. et al. Effect of angiogenic and antiangiogenic compounds on the outgrowth of capillary structures from fetal mouse bone explants. Lab. Invest. 81, 5–15 (2001).
Acknowledgements
We thank: H. Burchardt (Musculoskeletal Transplant Foundation) for advice with this research. J. Huard (University of Pittsburgh) for providing us with the sFlt-1 cDNA, W. Min (Yale University) for providing us with the Vegfa cDNA, Amgen Inc. for providing us with the OPG and Tnfsf11 cDNA and the RANK:Fc. We also thank C. Hock and D. Reynolds for assistance with the serum ELISA studies, B, Fan, L. Gehan and B. Stroyer for assistance with the histology, H. Awad for assistance with manuscript preparation, and R. Guldberg and A. Lin for μCT analyses. This work was supported by research grants from the Orthopedic Research and Education Foundation, the Musculoskeletal Transplant Foundation, US National Institutes of Health grants AR51469, AR48149, AR48681, AR43510, ES011854 and HL066973, and unrestricted research grants from DePuy, J&J Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J. Rabinowitz and R.J. Samulski are founders of Asklepios BioPharmaceutical, Inc. P.T. Rubery, R.J. O'Keefe and E.M. Schwarz are founding members of LAGeT, Inc.
Rights and permissions
About this article
Cite this article
Ito, H., Koefoed, M., Tiyapatanaputi, P. et al. Remodeling of cortical bone allografts mediated by adherent rAAV-RANKL and VEGF gene therapy. Nat Med 11, 291–297 (2005). https://doi.org/10.1038/nm1190
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1190
This article is cited by
-
Limited potential of AAV-mediated gene therapy in transducing human mesenchymal stem cells for bone repair applications
Gene Therapy (2021)
-
Current Trends in Viral Gene Therapy for Human Orthopaedic Regenerative Medicine
Tissue Engineering and Regenerative Medicine (2019)
-
“Ruffled border” formation on a CaP-free substrate: A first step towards osteoclast-recruiting bone-grafts materials able to re-establish bone turn-over
Journal of Materials Science: Materials in Medicine (2018)
-
Knockdown of toll-like receptor 4 signaling pathways ameliorate bone graft rejection in a mouse model of allograft transplantation
Scientific Reports (2017)
-
Enhancing the Functionality of Trabecular Allografts Through Polymeric Coating for Factor Loading
Regenerative Engineering and Translational Medicine (2017)