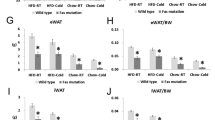
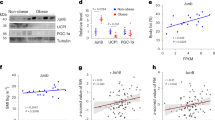
Abstract
Pten is an important phosphatase, suppressing the phosphatidylinositol-3 kinase/Akt pathway. Here, we generated adipose-specific Pten-deficient (AdipoPten-KO) mice, using newly generated Acdc promoter–driven Cre transgenic mice. AdipoPten-KO mice showed lower body and adipose tissue weights despite hyperphagia and enhanced insulin sensitivity with induced phosphorylation of Akt in adipose tissue. AdipoPten-KO mice also showed marked hyperthermia and increased energy expenditure with induced mitochondriagenesis in adipose tissue, associated with marked reduction of p53, inactivation of Rb, phosphorylation of cyclic AMP response element binding protein (CREB) and increased expression of Ppargc1a, the gene that encodes peroxisome proliferative activated receptor gamma coactivator 1 alpha. Physiologically, adipose Pten mRNA decreased with exposure to cold and increased with obesity, which were linked to the mRNA alterations of mitochondriagenesis. Our results suggest that altered expression of adipose Pten could regulate insulin sensitivity and energy expenditure. Suppression of adipose Pten may become a beneficial strategy to treat type 2 diabetes and obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Maehama, T. & Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 273, 13375–13378 (1998).
Jiang, G. & Zhang, B.B. Pi3-kinase and its up- and down-stream modulators as potential targets for the treatment of type II diabetes. Front. Biosci. 7, d903–d907 (2002).
Whiteman, E.L., Cho, H. & Birnbaum, M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 (2002).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr. Biol. 22, 1169–78 (1998).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Horie, Y. et al. Hepatocyte-specific Pten results in steatohepatitis and hepatocellular carcinomas. J. Clin. Invest. 113, 1774–1783 (2004).
Suzuki, A. et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 90, 657–667 (2003).
Kimura, T. et al. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 130, 1691–1700 (2003).
Ono, H. et al. Regulation of phosphoinositide metabolism, AKT phosphorylation, and glucose transport by PTEN (phosphatase and tensin homolog deleted on chromosome 10) in 3T3-L1 adipocytes. Mol. Endocrinol. 15, 1411–1422 (2001).
Nakashima, N. et al. The tumor suppressor PTEN negatively regulates insulin signaling in 3T3-L1 adipocytes. J. Biol. Chem. 275, 12889–12895 (2000).
Butler, M. et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes 51, 1028–1034 (2002).
Imai, T., Jiang, M., Chambon, P. & Metzger, D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor α mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc. Natl. Acad. Sci. USA 98, 224–228 (2001).
Bluher, M. et al. Adipose tissue selective insulin receptor knockout protects again obesity and obesity-related glucose intolerance. Dev. Cell. 3, 25–38 (2002).
Barlow, C. et al. Targeted expression of Cre recombinase to adipose tissue of transgenic mice directs adipose-specific excision of loxP-flanked gene segments. Nucleic Acid Res. 25, 2543–2545 (1997).
Fu, Y. et al. The adipocyte lipid binding protein (ALBP/aP2) gene facilitates foam cell formation in human THP-1 macrophages. Atherosclerosis 165, 259–269 (2002).
Boord, J.B., Fazio, S. & Linton, M.F. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr. Opin. Lipidol. 13, 141–147 (2002).
Weisberg, S.P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Maeda, K. et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (Adipose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289 (1996).
Okubo, K. et al. Large scale cDNA sequencing for analysis of quantitative and qualitative aspects of gene expression. Nat. Genet. 2, 173–179 (1992).
Scherer, P.E., Williams, S., Fogliano, M., Baldini, G. & Lodish, H.F. A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749 (1995).
Hu, E., Liang, P. & Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703 (1996).
Hotta, K. et al. Circulating concentrations of the adipocyte protein, adiponectin, are decreased in parallel with reduced insulin sensitivity during the progression to type-2 diabetes in rhesus monkeys. Diabetes 50, 1126–1133 (2001).
Kawamoto, S. et al. A novel reporter mouse that express enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 470, 263–268 (2000).
Hernandez, R., Teruel, T. & Lorenzo, M. Akt mediates insulin induction of glucose uptake and up-regulation of GLUT4 gene expression in brown adipocytes. FEBS Lett. 494, 225–231 (2001).
Yap, D.B., Hsieh, J.K., Chan, F.S. & Lu, X. mdm2: a bridge over the two tumour suppressors, p53 and Rb. Oncogene 18, 7681–7689 (1999).
Freeman, D.J. et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3, 117–130 (2003).
Hansen, J.B. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl. Acad. Sci. USA 101, 4112–4117 (2004).
Shreiber, S.N. et al. The estrogen-related receptorα (ERRα) functions in PPARγ coactivator 1α (PGC-1α)-induced mitochondrial biogenesis. Proc. Natl. Acad. Sci. USA 101, 6472–6477 (2004).
Stiles, B. et al. Liver-specific deletion of negative regulator PTEN results in fatty liver and insulin hypersensitivity. Proc. Natl. Acad. Sci. USA 101, 2082–2087 (2004).
Shimomura, I. et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol. Cell 6, 77–86 (2000).
Takahashi, M. et al. Genomic structure and mutations in adipose-specific gene, adiponectin. Int. J. Obes. Relat. Metab. Disord. 24, 861–868 (2000).
Iwaki, M. et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes 52, 1655–1663 (2003).
Tarutani, et al. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proc. Natl. Acad. Sci. USA 94, 7400–7405 (1997).
Miyawaki, K. et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat. Med. 8, 738–742 (2002).
Rossmeisl, M. et al. Expression of the uncoupling protein 1 from the aP2 gene promoter stimulates mitochondrial biogenesis in uniocular adipocytes in vivo. Eur. J. Biochem. 269, 19–28 (2002).
Maeda, N. et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 8, 731–737 (2002).
Nishizawa, H. et al. Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chem. 279, 19391–19395 (2004).
Kashiwagi, A. et al. The regulation of glucose transport by cAMP stimulators via three different mechanisms in rat and human adipocytes. J. Biol. Chem. 258, 13685–13692 (1983).
Acknowledgements
We thank Y. Matsuzawa and K. Sugihara for their encouragement, and K. Nishida, K. Oiki and S. Mizuno for technical assistance. This work was supported in part by grants from the Suzuken Memorial Foundation, The Nakajima Foundation, Kanae Foundation for Life and Socio-Medical Science, The Tokyo Biochemical Research Foundation, Takeda Medical Research Foundation, Uehara Memorial Foundation, Takeda Science Foundation, Novartis Foundation (Japan) for the Promotion of Science, The Cell Science Research Foundation, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, a Grant-in-Aid from the Japan Medical Association, The Naito Foundation, a grant from the Japan Heart Foundation Research, Kato Memorial Bioscience Foundation, Japan Research Foundation for Clinical Pharmacology, a grant from the Ministry of Health, Labor and Welfare, Japan, and Grants-in-Aid from COE Research and Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Expression analyses of Cre in Adiponectin promoter-driven Cre transgenic mice. (PDF 34 kb)
Supplementary Fig. 2
Supplemental data of Fig. 4b, 5b, 5d and 5e. (PDF 286 kb)
Supplementary Table 1
Primers used in mRNA analyses (PDF 20 kb)
Rights and permissions
About this article
Cite this article
Komazawa, N., Matsuda, M., Kondoh, G. et al. Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific Pten suppression in mice. Nat Med 10, 1208–1215 (2004). https://doi.org/10.1038/nm1117
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1117
This article is cited by
-
TNF-α induces vascular insulin resistance via positive modulation of PTEN and decreased Akt/eNOS/NO signaling in high fat diet-fed mice
Cardiovascular Diabetology (2016)
-
Selective enhancement of insulin sensitivity in the mature adipocyte is sufficient for systemic metabolic improvements
Nature Communications (2015)
-
Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2
Molecular Cancer (2013)
-
Correction: RETRACTION: Enhanced insulin sensitivity, energy expenditure and thermogenesis in adipose-specific Pten suppression in mice
Nature Medicine (2005)
-
The transcription factor GATA2 regulates differentiation of brown adipocytes
EMBO reports (2005)