Abstract
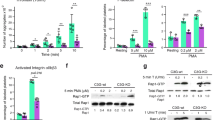
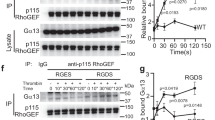
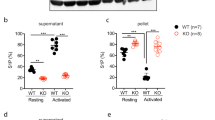
Signaling through the second messengers calcium and diacylglycerol (DAG) is a critical element in many biological systems. Integration of calcium and DAG signals has been suggested to occur primarily through protein kinase C family members, which bind both calcium and DAG. However, an alternative pathway may involve members of the CalDAG-GEF/RasGRP protein family, which have structural features (calcium-binding EF hands and DAG-binding C1 domains) that suggest they can function in calcium and DAG signal integration1,2. To gain insight into the signaling systems that may be regulated by CalDAG-GEF/RasGRP family members, we have focused on CalDAG-GEFI, which is expressed preferentially in the brain and blood1. Through genetic ablation in the mouse, we have found that CalDAG-GEFI is crucial for signal integration in platelets. Mouse platelets that lack CalDAG-GEFI are severely compromised in integrin-dependent aggregation as a consequence of their inability to signal through CalDAG-GEFI to its target, the small GTPase Rap1. These results suggest that analogous signaling defects are likely to occur in the central nervous system when CalDAG-GEFI is absent or compromised in function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kawasaki, H. et al. A Rap guanine nucleotide exchange factor enriched highly in the basal ganglia. Proc. Natl. Acad. Sci. USA 95, 13278–13283 (1998).
Ebinu, J.O. et al. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Science 280, 1082–1086 (1998).
Bos, J.L. et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem. Soc. Trans. 31, 83–86 (2003).
Bertoni, A. et al. Relationships between Rap1b, affinity modulation of integrin αIIbβ3, and the actin cytoskeleton. J. Biol. Chem. 277, 25715–25721 (2002).
Eto, K. et al. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc. Natl. Acad. Sci. USA 99, 12819–12824 (2002).
de Bruyn, K.M., Zwartkruis, F.J., de Rooij, J., Akkerman, J.W. & Bos, J.L. The small GTPase Rap1 is activated by turbulence and is involved in integrin αIIbβ3-mediated cell adhesion in human megakaryocytes. J. Biol. Chem. 278, 22412–22417 (2003).
Franke, B., Akkerman, J.W. & Bos, J.L. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 16, 252–259 (1997).
Franke, B. et al. Sequential regulation of the small GTPase Rap1 in human platelets. Mol. Cell. Biol. 20, 779–785 (2000).
Newman, P.J., Seligsohn, U., Lyman, S. & Coller, B.S. The molecular genetic basis of Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations in Israel. Proc. Natl. Acad. Sci. USA 88, 3160–3164 (1991).
Bergmeier, W. et al. Flow cytometric detection of activated mouse integrin αIIbβ3 with a novel monoclonal antibody. Cytometry 48, 80–86 (2002).
Klages, B., Brandt, U., Simon, M.I., Schultz, G. & Offermanns, S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J. Cell Biol. 144, 745–754 (1999).
Nieswandt, B. et al. Glycoprotein VI but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 20, 2120–2130 (2001).
Savage, B., Almus-Jacobs, F. & Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 94, 657–666 (1998).
Davis, R.E., Stenberg, P.E., Levin, J. & Beckstead, J.H. Localization of megakaryocytes in normal mice and following administration of platelet antiserum, 5-fluorouracil, or radiostrontium: evidence for the site of platelet production. Exp. Hematol. 25, 638–648 (1997).
Ebinu, J.O. et al. RasGRP links T-cell receptor signaling to Ras. Blood 95, 3199–3203 (2000).
Teixeira, C., Stang, S.L., Zheng, Y., Beswick, N.S. & Stone, J.C. Integration of DAG signaling systems mediated by PKC-dependent phosphorylation of RasGRP3. Blood 102, 1414–1420 (2003).
Yamashita, S. et al. CalDAG-GEFIII activation of Ras, R-ras, and Rap1. J. Biol. Chem. 275, 25488–25493 (2000).
Yang, Y. et al. RasGRP4, a new mast cell-restricted Ras guanine nucleotide-releasing protein with calcium- and diacylglycerol-binding motifs. Identification of defective variants of this signaling protein in asthma, mastocytosis, and mast cell leukemia patients and demonstration of the importance of RasGRP4 in mast cell development and function. J. Biol. Chem. 277, 25756–25774 (2002).
Lova, P., Paganini, S., Sinigaglia, F., Balduini, C. & Torti, M. A Gi-dependent pathway is required for activation of the small GTPase Rap1B in human platelets. J. Biol. Chem. 277, 12009–12015 (2002).
Woulfe, D., Jiang, H., Mortensen, R., Yang, J. & Brass, L.F. Activation of Rap1B by Gi family members in platelets. J. Biol. Chem. 277, 23382–23390 (2002).
Larson, M.K. et al. Identification of P2Y12-dependent and -independent mechanisms of glycoprotein VI-mediated Rap1 activation in platelets. Blood 101, 1409–1415 (2003).
Herrmann, C., Horn, G., Spaargaren, M. & Wittinghofer, A. Differential interaction of the ras family GTP-binding proteins H-Ras, Rap1A, and R-Ras with the putative effector molecules Raf kinase and Ral-guanine nucleotide exchange factor. J. Biol. Chem. 271, 6794–6800 (1996).
Katagiri, K., Maeda, A., Shimonaka, M. & Kinashi, T. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4, 741–748 (2003).
Bergmeier, W. et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood 102, 4229–4235 (2003).
Guo, F., Kumahara, E. & Saffen, D. A CalDAG-GEFI/Rap1/B-Raf cassette couples M1 muscarinic acetylcholine receptors to the activation of ERK1/2. J. Biol. Chem. 276, 25568–25581 (2001).
Acknowledgements
We are grateful to B. Roe for providing a BAC clone of CalDAG-GEFI; T. Kinashi for providing antibodies to RapL; M. Yaffe for critical reading of the manuscript; R. Shivdasani and R. Hynes for advice; and P. Harlan for technical support. This work was funded by National Institute of Child Health and Development grant R01-HD28341 (A.M.G.); the James and Pat Poitras Research Fund (A.M.G.); the Grace B. Kerr Fund (A.M.G.); the Angus & Monaise MacDonald Fund (A.M.G.); National Institutes of Mental Health grant F32-MH065815 (J.R.C.); a grant from the Deutsche Forschungsgemeinschaft (W.B.); and the National Heart, Lung, and Blood Institute of the National Institutes of Health grants P01-HL56949 (D.D.W.) and HL66105 (D.E.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The Massachusetts Institute of Technology has filed a patent application on behalf of J.R.C., A.M.G. and D.E.H., “The Use of Protein Inhibitors as Antithrombotic Agents,” case no. 10198 filed with the US Patent Office on June 2, 2003. The protein referred to in the patent application is that described in this manuscript, CalDAG-GEFI.
Supplementary information
Supplementary Fig. 1
Aggregation assays in response to calcium ionophore A23187. (PDF 643 kb)
Supplementary Table 1
Clotting factor assays in CalDAG-GEFT−/− mice. (PDF 17 kb)
Supplementary Table 2
Blood cell counts in CalDAG-GEFT−/− mice. (PDF 24 kb)
Supplementary Video 1
Platelets in whole blood from CalDAG-GEFI knockout mice do not form thrombi on a collagen surface. Real-time movies of platelets perfused over a collagen plate. a, wild-type calcein-labeled platelets, and b, CalDAG-GEFI−/− calcein-labeled platelets. (MOV 2658 kb)
Rights and permissions
About this article
Cite this article
Crittenden, J., Bergmeier, W., Zhang, Y. et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med 10, 982–986 (2004). https://doi.org/10.1038/nm1098
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1098
This article is cited by
-
Platelet C3G: a key player in vesicle exocytosis, spreading and clot retraction
Cellular and Molecular Life Sciences (2024)
-
Crk proteins activate the Rap1 guanine nucleotide exchange factor C3G by segregated adaptor-dependent and -independent mechanisms
Cell Communication and Signaling (2023)
-
Rap1 in the Context of PCSK9, Atherosclerosis, and Diabetes
Current Atherosclerosis Reports (2023)
-
Influence of genetic polymorphisms in P2Y12 receptor signaling pathway on antiplatelet response to clopidogrel in coronary heart disease
BMC Cardiovascular Disorders (2022)
-
Phosphatidic acid-dependent localization and basal de-phosphorylation of RA-GEFs regulate lymphocyte trafficking
BMC Biology (2020)