Abstract
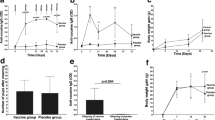
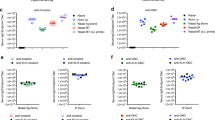
Cocaine abuse is a major medical and public health concern in the United States, with approximately 2.1 million people dependent on cocaine1. Pharmacological approaches to the treatment of cocaine addiction have thus far been disappointing2,3, and new therapies are urgently needed. This paper describes an immunological approach to cocaine addiction. Antibody therapy for neutralization of abused drugs has been described previously4, including a recent paper demonstrating the induction of anti–cocaine antibodies5. However, both the rapidity of entry of cocaine into the brain6 and the high doses of cocaine frequently encountered7 have created challenges for an antibody–based therapy. Here we demonstrate that antibodies are efficacious in an animal model of addiction. Intravenous cocaine self–administration in rats was inhibited by passive transfer of an anti–cocaine monoclonal antibody. To actively induce anti–cocaine antibodies, a cocaine vaccine was developed that generated a high–titer, long–lasting antibody response in mice. Immunized mice displayed a significant change in cocaine pharmacokinetics, with decreased levels of cocaine measured in the brain of immunized mice only 30 seconds after intravenous (i.v.) administration of cocaine. These data establish the feasibility of a therapeutic cocaine vaccine for the treatment of cocaine addiction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Institute of Medicine. Development of Medications for the Treatment of Opiate and Cocaine Addictions: Issues for the Government and Private Sector. (National Academy Press, Washington, DC, 1995).
Carroll, F.I., Lewin, A.H. & Biswas, J. Chemical approaches to the treatment of cocaine abuse. Pharm. News 1, 11–16 (1994).
Bonese, K.F., Wainer, B.H., Fitch, F.W., Rothberg, R.M. & Schuster, C.R. Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature 252, 708–710 (1974).
Carrera, M.R.A., Ashley, J.A., Parsons, L.H., Wirsching, P., Koob, G.F. & Janda, K.D. Suppression of psychoactive effects of cocaine by active immunization. Nature 378, 727–730 (1995).
Nutt, D.J. Addiction: Brain mechanisms and their treatment implications. Lancet 347, 31–36 (1996).
Johanson, C.-E. & Fischman, M.W. The pharmacology of cocaine related to its abuse. Pharmacol. Rev. 41, 3–52 (1989).
Katz, J. & Goldberg, S.R. Second-order schedules of drug injection. in Methods of Assessing the Reinforcing Properties of Abused Drugs. (ed. Bosarth, M.) 105–111 (Springer, New York, 1987).
Bergman, J., Kamien, J.B. & Spealman, R.D. Antagonism of cocaine self-administration by selective dopamine Dl D2 antagonists. Behav. Pharmacol. 1, 355–363 (1990).
Woods, J.H., Winger, G.D. & France, C.P. Reinforcing and discriminative stimulus effects of cocaine: Analysis of pharmacological mechanisms. in Cocaine: Clinical and Biobehavioral Aspects. (eds. Fisher, S., Raskin, A. & Uhlenhuth, E.) 21–65 (Oxford Univ. Press, New York, 1987).
Goldberg, S.R., Spealman, R.D., Risner, M.F. & Henningfield, J.E. Control of behavior by intravenous nicotine injections in laboratory animals. Pharmacol. Biochem. Behav. 19, 1011–1020 (1983).
Bodanszky, M. The myth of coupling reagents. Peptide Res. 5, 134–139 (1992).
Jindal, S.P. & Lutz, T. Mass spectrometric studies of cocaine disposition in animals and humans using stable isotope-labeled analogues. J. Pharm. Sci. 78, 1009–1014 (1989).
Jeffcoat, A.R., Perez-Reyes, M., Hill, J.M., Sadler, B.M. & Cook, C.E. Cocaine disposition in humans after intravenous injection, nasal insufflation (snorting), or smoking. Drug Metab. Disposition 17, 153–159 (1989).
Jatlow, P. et al. Cocaethylene: A neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 48, 1787–1794 (1991).
Steward, M.W. Overview: Introduction to methods used to study the affinity and kinetics of antibody-antigen reactions. in Handbook of Experimental Immunology, Vol. 1, Immunochemistry. (ed. Weir, D.M.) 25.1–25.30 (Blackwell Scientific, Oxford, 1986).
Jatlow, P.J. Cocaine: Analysis, pharmacokinetics, and metabolic disposition. Yale J. Biol. Med. 61, 105–113 (1988).
Benowitz, N.L. Clinical pharmacology and toxicology of cocaine. Pharmacol. Toxicol. 72, 3–12 (1993).
Oldendorf, W.H. Some relationships between addiction and drug delivery to the brain. in Bioavailability of Drugs to the Brain and the Blood-Brain Barrier. (eds. Frankenheim, J. & Brown, R.M.) NIDA Res. Monogr. 120, 13–25 (1992).
Kirchner, J.G. Thin-layer chromatography. Tech. Chem. 14, 736–737 (1978).
Kantak, K.M., Wasserman, S.J., Lawley, S.I. & O'Connor, T. Acute and multiple injection effects of magnesium on responding maintained by cocaine, extinction from cocaine, glucose + saccharin, and food. Pharmacol. Biochem. Behav. 41, 415–423 (1992).
Rothstein, T.L. & Gefter, M.L. Affinity analysis of idiotype-positive and idiotype-negative Ars-binding hybridoma proteins and Ars-immune serum. Mol. Immunol. 20, 161–168 (1983).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fox, B., Kantak, K., Edwards, M. et al. Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 2, 1129–1132 (1996). https://doi.org/10.1038/nm1096-1129
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1096-1129
This article is cited by
-
The M3-TT Vaccine Decreases the Antinociceptive Effects of Morphine and Heroin in Mice
International Journal of Mental Health and Addiction (2023)
-
Novel Pharmacological Agents for the Treatment of Cocaine Use Disorder
Current Behavioral Neuroscience Reports (2022)
-
Novel mucosal adjuvant, mastoparan-7, improves cocaine vaccine efficacy
npj Vaccines (2020)
-
Effects of a methamphetamine vaccine, IXT-v100, on methamphetamine-related behaviors
Psychopharmacology (2020)
-
Ethical Implications in Vaccine Pharmacotherapy for Treatment and Prevention of Drug of Abuse Dependence
Journal of Bioethical Inquiry (2018)