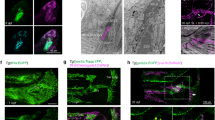
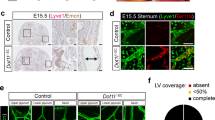
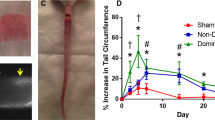
Abstract
Lymphatic vessels are essential for the removal of interstitial fluid and prevention of tissue edema. Lymphatic capillaries lack associated mural cells, and collecting lymphatic vessels have valves, which prevent lymph backflow. In lymphedema-distichiasis (LD), lymphatic vessel function fails because of mutations affecting the forkhead transcription factor FOXC2. We report that Foxc2−/− mice show abnormal lymphatic vascular patterning, increased pericyte investment of lymphatic vessels, agenesis of valves and lymphatic dysfunction. In addition, an abnormally large proportion of skin lymphatic vessels was covered with smooth muscle cells in individuals with LD and in mice heterozygous for Foxc2 and for the gene encoding lymphatic endothelial receptor, Vegfr3 (also known as Flt4). Our data show that Foxc2 is essential for the morphogenesis of lymphatic valves and the establishment of a pericyte-free lymphatic capillary network and that it cooperates with Vegfr3 in the latter process. Our results indicate that an abnormal interaction between the lymphatic endothelial cells and pericytes, as well as valve defects, underlie the pathogenesis of LD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Irrthum, A., Karkkainen, M.J., Devriendt, K., Alitalo, K. & Vikkula, M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am. J. Hum. Genet. 67, 295–301 (2000).
Karkkainen, M.J. et al. Missense mutations interfere with VEGFR-3 signaling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Irrthum, A. et al. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am. J. Hum. Genet. 72, 1470–1478 (2003).
Fang, J. et al. Mutations in FOXC2 (MFH-1), a forkhead family transcription factor, are responsible for the hereditary lymphedema-distichiasis syndrome. Am. J. Hum. Genet. 67, 1382–1388 (2000).
Finegold, D.N. et al. Truncating mutations in FOXC2 cause multiple lymphedema syndromes. Hum. Mol. Genet. 10, 1185–1189 (2001).
Bell, R. et al. Analysis of lymphoedema-distichiasis families for FOXC2 mutations reveals small insertions and deletions throughout the gene. Hum. Genet. 108, 546–551 (2001).
Brice, G. et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. J. Med. Genet. 39, 478–483 (2002).
Iida, K. et al. Essential roles of the winged helix transcription factor MFH-1 in aortic arch patterning and skeletogenesis. Development 124, 4627–4638. (1997).
Winnier, G.E., Hargett, L. & Hogan, B.L. The winged helix transcription factor MFH1 is required for proliferation and patterning of paraxial mesoderm in the mouse embryo. Genes Dev. 11, 926–940 (1997).
Winnier, G.E. et al. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev. Biol. 213, 418–431 (1999).
Kriederman, B.M. et al. FOXC2 haploinsufficient mice are a model for human autosomal dominant lymphedema-distichiasis syndrome. Hum. Mol. Genet. 12, 1179–11785 (2003).
Karkkainen, M.J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Ozerdem, U., Grako, K.A., Dahlin-Huppe, K., Monosov, E. & Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 222, 218–227 (2001).
Lindahl, P., Johansson, B.R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Bondjers, C. et al. Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth Muscle cells. Am. J. Pathol. 162, 721–729 (2003).
Li, D.Y. et al. Defective angiogenesis in mice lacking endoglin. Science 284, 1534–1537 (1999).
Oliver, G. & Detmar, M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 16, 773–783 (2002).
Wigle, J.T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Wigle, J.T. et al. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 21, 1505–1513 (2002).
Kaipainen, A. et al. Expression of the fms-like tyrosine kinase FLT4 gene becomes restricted to lymphatic endothelium during development. Proc. Natl. Acad. Sci. USA 92, 3566–3570 (1995).
Dumont, D.J. et al. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282, 946–949 (1998).
Saaristo, A. et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J. Exp. Med. 196, 719–730 (2002).
Kume, T., Jiang, H., Topczewska, J.M. & Hogan, B.L. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 15, 2470–2482 (2001).
Kaestner, K.H. et al. Clustered arrangement of winged helix genes fkh-6 and MFH-1: possible implications for mesoderm development. Development 122, 1751–1758 (1996).
Miura, N., Iida, K., Kakinuma, H., Yang, X.L. & Sugiyama, T. Isolation of the mouse (MFH-1) and human (FKHL 14) mesenchyme fork head-1 genes reveals conservation of their gene and protein structures. Genomics 41, 489–492. (1997).
Gnepp, D.R. & Green, F.H.Y. Scanning electron microscopic study of canine lymphatic vessels and their valves. Lymphology 13, 91–99 (1980).
Kume, T., Deng, K. & Hogan, B.L. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127, 1387–1395 (2000).
Gerhardt, H. & Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 314, 15–23 (2003).
Hirschi, K.K. & D'Amore, P.A. Pericytes in the microvasculature. Cardiovasc. Res. 32, 687–698 (1996).
Carmeliet, P. et al. Role of tissue factor in embryonic blood vessel development. Nature 383, 73–75 (1996).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1161–1169 (1997).
Yang, X. et al. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development 126, 1571–1580 (1999).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000).
Oh, S.P. et al. Activin receptor-like kinase 1 modulates transforming growth factor-β1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 97, 2626–2631 (2000).
Gale, N.W. et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell 3, 411–423 (2002).
Petrova, T.V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Hirakawa, S. et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am. J. Pathol. 162, 575–586 (2003).
Yang, X.L., Matsuura, H., Fu, Y., Sugiyama, T. & Miura, N. MFH-1 is required for bone morphogenetic protein-2-induced osteoblastic differentiation of C2C12 myoblasts. FEBS Lett. 470, 29–34 (2000).
Taylor, L.M. & Khachigian, L.M. Induction of platelet-derived growth factor B-chain expression by transforming growth factor-β involves transactivation by Smads. J. Biol. Chem. 275, 16709–16716 (2000).
Vajda, J. & Tomcsik, M. The structure of the valves of the lymphatic vessels. Acta Anat. 78, 521–531 (1971).
Veikkola, T. et al. Intrinsic versus microenvironmental regulation of lymphatic endothelial cell phenotype and function. FASEB J. 17, 2006–2013 (2003).
Karkkainen, M.J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 98, 12677–12682 (2001).
Furumoto, T.A. et al. Notochord-dependent expression of MFH1 and PAX1 cooperates to maintain the proliferation of sclerotome cells during the vertebral column development. Dev. Biol. 210, 15–29 (1999).
Breiteneder-Geleff, S. et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 154, 385–394 (1999).
Laakkonen, P., Porkka, K., Hoffman, J.A. & Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 8, 751–755 (2002).
Wilkinson, D.G. (ed.). In Situ Hybridization: A Practical Approach (Oxford University Press, Oxford, UK, 1998).
Yamaguchi, T., Dumont, D., Conion, R., Breitman, M. & Rossant, J. Flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development 118, 489–498 (1993).
Laitinen, M. et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum. Gene Ther. 9, 1481–1486 (1998).
Lennon, G., Auffray, C., Polymeropoulos, M., Soares, M.B. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics 33, 151–152 (1996).
Makinen, T. et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO. J. 20, 4762–4773 (2001).
Acknowledgements
We thank St George's and St Thomas' lymphedema consortium (S. Jeffery, J.R. Levick, A. Child, K.G. Burnand, S. Mansour, G. Brice, M. Sarfarazi and C. Sholto-Douglas-Vernon) for help with the patient material; C. Betsholtz and C. Bondjers for providing probes for Pdgfb, Pdgfrb, Rgs5 and for discussions; P. Laakkonen and M. Jeltsch for providing anti-Lyve-1 sera; U. Ozerdem for providing the NG2 antibodies; M. Kimak for the preparation of DNA from individuals with LD; and T. Tainola, A. Parsons, M. Helanterä, T. Laakkonen, S. Lampi, K. Makkonen, P. Hyvärinen and M. Miller for technical assistance. This study was supported by grants from Finnish Academy of Sciences, Finnish Cancer Organizations, Sigrid Juselius Foundation, Helsinki University Central Hospital, Finnish Cultural Foundation (T.K.), Paulo Foundation (T.K.), Emil Aaltonen Foundation (T.K.), Aarne Koskelo Foundation (T.K.) and by the EU Integrated Project LSHG-CT-2004-503573.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Analysis of lymphatic vasculature in adult Foxc2+/− mice. (PDF 310 kb)
Supplementary Fig. 2
Normal early development of lymphatic vasculature in Foxc2−/− mice. (PDF 506 kb)
Supplementary Fig. 3
Abnormal lymphatic patterning and PC/SMC recruitment in the skin of Foxc2−/− embryos. (PDF 295 kb)
Supplementary Fig. 4
Skin blood vessel development is normal in Foxc2−/− mice. (PDF 378 kb)
Supplementary Fig. 5
Mural cells associated with lymphatic vessels of Foxc2−/− mice express the PC marker NG2. (PDF 580 kb)
Supplementary Fig. 6
Pdgfb, Vegfr3, Prox1 and podoplanin are expressed in lymphatic endothelium lacking Foxc2. (PDF 320 kb)
Supplementary Fig. 7
Genetic relationship between Vegfr3 and Foxc2. (PDF 309 kb)
Supplementary Fig. 8
Expression pattern of FOXC2. (PDF 250 kb)
Supplementary Table 1
Use of antibodies against lymphatic endothelial specific markers in different experimental settings. (PDF 19 kb)
Rights and permissions
About this article
Cite this article
Petrova, T., Karpanen, T., Norrmén, C. et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10, 974–981 (2004). https://doi.org/10.1038/nm1094
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1094
This article is cited by
-
A Prox1 enhancer represses haematopoiesis in the lymphatic vasculature
Nature (2023)
-
Meningeal lymphatic vessels mediate neurotropic viral drainage from the central nervous system
Nature Neuroscience (2022)
-
A family with Milroy disease caused by the FLT4/VEGFR3 gene variant c.2774 T > A
BMC Medical Genomics (2021)
-
The lymphatics in kidney health and disease
Nature Reviews Nephrology (2021)
-
Primary lymphoedema
Nature Reviews Disease Primers (2021)