Abstract
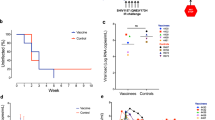
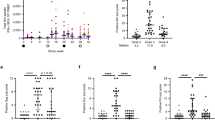
Prolonged antiretroviral therapy (ART) is not likely to eradicate human immunodeficiency virus type I (HIV-I) infection. Here we explore the effect of therapeutic immunization in the context of ART during primary infection using the simian immunodeficiency virus (SIV251) macaque model. Vaccination of rhesus macaques with the highly attenuated poxvirus-based NYVAC-SIV vaccine expressing structural genes elicited vigorous virus-specific CD4+ and CD8+ T cell responses in macaques that responded effectively to ART. Following discontinuation of a six-month ART regimen, viral rebound occurred in most animals, but was transient in six of eight vaccinated animals. Viral rebound was also transient in four of seven mock-vaccinated control animals. These data establish the importance of antiretroviral treatment during primary infection and demonstrate that virus-specific immune responses in the infected host can be expanded by therapeutic immunization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Palella, F.J. Jr. et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N. Engl. J. Med. 338, 853–860 (1998).
Finzi, D. et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997).
Wong, J.K. et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278, 1291– 1295 (1997).
Davey, R.T. Jr. et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl Acad. Sci. USA 96, 15109–15114 (1999).
Lisziewicz, J. et al. Control of HIV despite the discontinuation of antiretroviral therapy. N. Engl. J. Med. 340, 1683– 1684 (1999).
Ortiz, G.M. et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J. Clin. Invest. 104, R13– R18 (1999).
Borrow, P., Lewicki, H., Hahn, B.H., Shaw, G.M. & Oldstone, M.B. A. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68, 6103 –6110 (1994).
Koup, R.A. et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome . J. Virol. 68, 4650–4655 (1994).
Nixon, D.F. et al. HIV-1 Gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336, 484–487 (1988).
Pantaleo, G. et al. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature 370, 463–467 (1994).
Rinaldo, C. et al. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69, 5838–5842 ( 1995).
Autran, B. et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277, 112–116 ( 1997).
Oxenius, A. et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl Acad. Sci. USA 97, 3382– 3387 (2000).
Rosenberg, E.S. et al. Vigorous HIV-1-specific CD4+ t cell responses associated with control of viremia. Science 278, 1447– 1450 (1997).
Gray, C.M. et al. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART). J. Immunol. 162, 1780–1788 (1999).
Morris, L. et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188, 233–245 (1998).
Ogg, G.S. et al. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J. Virol. 73, 797–800 ( 1999).
Benson, J. et al. Recombinant vaccine-induced protection against the highly pathogenic SIV mac251: dependence on route of challenge exposure . J. Virol. 72, 4170–4182 (1998).
Evans, T.G. et al. A canarypox vaccine expressing multiple human immunodeficiency virus type 1 genes given alone or with rgp120 elicits broad and durable CD8+ cytotoxic T lymphocyte responses in seronegative volunteers. J. Infect. Dis. 180, 290–298 (1999).
Hanke, T. et al. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus ankara boost vaccination regimen. J. Virol. 73, 7524–7532 (1999).
Seth, A. et al. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74, 2502– 2509 (2000).
Franchini, G. et al. Highly attenuated HIV type 2 recombinant poxviruses, but not HIV-2 recombinant Salmonella vaccines, induce long-lasting protection in rhesus macaques. AIDS Res. Hum. Retroviruses 11, 909–920 (1995).
Klein, J. et al. Nomenclature for the major histocompatibility complexes of different species: a proposal. Immunogenetics 31, 217–219 (1990).
Knapp, L.A., Lehmann, E., Piekarczyk, M.S., Urvater, J.A. & Watkins, D.I. A high frequency of Mamu-A*01 in the rhesus macaque detected by PCR-SSP and direct sequencing. Tissue Antigens 50, 657–661 (1997).
Allen, T.M. et al. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160, 6062–6071 (1998).
Miller, M.D., Yamamoto, H., Hughes, A.L., Watkins, D.I. & Letvin, N.L. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147, 320–329 (1991).
Franchini, G. et al. Sequence of simian immunodeficiency virus and its relationship to the human immunodeficiency viruses. Nature 328, 539–543 (1987).
Letvin, N.L. et al. The CD8+ T lymphocyte response during primary SIVmac infection . Adv. Exp. Med. Biol. 452, 177– 179 (1998).
Binley, J.M. et al. The relationship between T cell proliferative responses and plasma viremia during treatment of human immunodeficiency virus type 1 infection with combination antiretroviral therapy. J. Infect. Dis. 181, 1249–1263 (2000).
Haslett, P.A. et al. Strong human immunodeficiency virus (HIV)-specific CD4+ T cell responses in a cohort of chronically infected patients are associated with interruptions in anti-HIV chemotherapy. J. Infect. Dis. 181, 1264–1272 (2000).
Herbein, G. et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395, 189–194 ( 1998).
Li, C.J., Friedman, D.J., Wang, C., Metelev, V. & Pardee, A.B. Induction of apoptosis in uninfected lymphocytes by HIV-1. Science 268, 429– 431 (1995).
Westendorp, M.O. et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 . Nature 375, 497–500 (1995).
Pitcher, C.J. et al. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression . Nature Med. 5, 518–525 (1999).
Yasutomi, Y. et al. A vaccine-elicited, single viral epitope-specific cytotoxic T lymphocyte response does not protect against intravenous, cell-free simian immunodeficiency virus challenge. J. Virol. 69, 2279–2284 (1995).
Allen, T.M. et al. Acute viral escape from cytotoxic T lymphocytes: implications for HIV vaccine design. Nature (in press).
Tsai, C.-C. et al. Prevention of simian immunodeficiency virus infection in macaques by 9-(2-phosphonylmethoxypropyl) adenine (PMPA). Science 270, 1197–1199 (1995).
Tartaglia, J. et al. NYVAC: a highly attenuated strain of vaccinia virus. Virology 188, 217–232 ( 1992).
Barouch, D.H. et al. Augmentation of immune responses to HIV-1 and simian immunodeficiency virus DNA vaccines by IL-2/Ig plasmid administration in rhesus monkeys. Proc. Natl Acad. Sci. USA 97, 4192– 4197 (2000).
Jacobson, E.L., Pilaro, F. & Smith, K.A. Rational interleukin 2 therapy for HIV positive individuals: daily low doses enhance immune function without toxicity . Proc. Natl Acad. Sci. USA 93, 10405– 10410 (1996).
Kovacs, J.A. et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N. Engl. J. Med. 335, 1350–1356 (1996).
Romano, J.W., Williams, K.G., Shurtliff, R.N., Ginocchio, C. & Kaplan, M. NASBA technology: isothermal RNA amplification in qualitative and quantitative diagnostics. Immunol. Invest. 26, 15–28 (1997).
Acknowledgements
We thank R. Klausner, M. Klein, R. Yarchoan and R. Little for discussion; R. Pal for the SIV251 stock; C. Mclaren for Stavudine; X. Wang for making the tetramers; the ABL staff for animal care; and S. Snodgrass for editorial assistance. M.Poudyal. was supported by the National Foundation for Biomedical Research as a Clinical Research Training Program Scholar.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hel, Z., Venzon, D., Poudyal, M. et al. Viremia control following antiretroviral treatment and therapeutic immunization during primary SIV251 infection of macaques. Nat Med 6, 1140–1146 (2000). https://doi.org/10.1038/80481
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/80481
This article is cited by
-
Prolonged tenofovir treatment of macaques infected with K65R reverse transcriptase mutants of SIV results in the development of antiviral immune responses that control virus replication after drug withdrawal
Retrovirology (2012)
-
SIV antigen immunization induces transient antigen-specific T cell responses and selectively activates viral replication in draining lymph nodes in retroviral suppressed rhesus macaques
Retrovirology (2011)
-
Moving candidate vaccines into development from research: lessons from HIV
Immunology & Cell Biology (2009)
-
Effect of therapeutic immunization using Ad5/35 and MVA vectors on SIV infection of rhesus monkeys undergoing antiretroviral therapy
Gene Therapy (2009)
-
CD8+ Cell Anti-HIV Activity Rapidly Increases Upon Discontinuation of Early Antiretroviral Therapy
Journal of Clinical Immunology (2009)