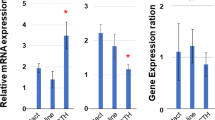
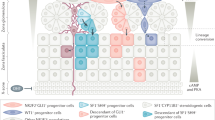
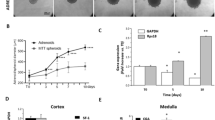
Abstract
Xenotransplanted adrenocortical tissue of clonal origin was formed in immunodeficient (scid) mice by using techniques of cell transplantation. The experiments reported here used a single clone of bovine adrenocortical cells, but 5 of 20 other randomly selected clones also formed tissue. Most adrenalectomized animals bearing transplanted cells survived indefinitely, demonstrating that the cells restored the animals' capacity to survive in the absence of sodium supplementation. Formation of well-vascularized tissue at the site of transplantation was associated with stable levels of cortisol in the blood, replacing the mouse glucocorticoid (corticosterone). Ultrastructurally, the cultured cells before transplantation had characteristics of rapidly growing cells, but tissue formed in vivo showed features associated with active steroidogenesis. These experiments show that an endocrine tissue can be derived from a single, normal somatic cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Langer, R. & Vacanti, J.P. Tissue engineering. Science 260, 920–926 (1993).
Winn, S.R. et al. Polymer-encapsulated cells genetically modified to secrete human nerve growth factor promote the survival of axotomized septal cholinergic neurons. Proc. Notl. Acad. Sci. USA 91, 2324–2328 (1994).
Vogt, P.M. et al. Genetically modified keratinocytes transplanted to wounds reconstitute the epidermis. Proc. Notl. Acad. Sci. USA 91, 9307–9311 (1994).
Pavlath, G.K., Rando, T.A. & Blau, H.M. Transient immunosuppressive treatment leads to long-term retention of allogeneic myoblasts in hybrid myofibers. J. Cell Biol. 127, 1923–1932 (1994).
Crane, G.M., Ishaug, S.L. & Mikos, A.G. Bone tissue engineering. Nature Med. 1, 1322–1324 (1995).
Moullier, P., Bohl, D. Cardoso J., Heard, J. M. . & Danos, O. Long-term delivery of a lysosomal enzyme by genetically modified fibroblasts in dogs. Nature Med. 1, 353–357 (1995).
Aebischer, P. et al. Intrathecal deliveiy of CNTF using encapsulated genetically modified xenogeneic cells in amyotrophic lateral sclerosis patients. Nature Med. 2, 696–699 (1996).
Lacorazza, H.D., Flax J.D., Snyder, E. Y. & Jendoubi M. Expression of human β-hexosaminidase α-subunit gene (the gene defect of Tay-Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nature Med. 2, 424–429 (1996).
Hornsby, P.J. & McAllister, J.M. Culturing steroidogenic cells, in: Methods in Enzymology. 206. (eds. Waterman, M. R. St Johnson, E. F.) 371–380 (Academic, San Diego, 1991).
Hornsby, P.J. et al. Changes in gene expression during senescence of adrenocortical cells in culture. J. Steroid Biochem. Mol. Biol. 43, 385–395 (1992).
Hornsby, P.J. Culturing steroidogenic cells in: Cell and Tissue Culture: Laboratory Procedures, 17B (eds. Griffiths, J. B., Doyle, A. St Newell, D. G.) 7.1–7.9 (Wiley, Chichester, UK, 1994).
Hornsby, P.J., Aldern, K.A. & Harris, S.E. Clonal variation in response to adrenocorticotropin in cultured bovine adrenocortical cells: Relationship to senescence. J. Cell. Physiol. 129, 395–402 (1986).
Hornsby, P.J. et al. Loss of expression of a differentiated function gene, steroid 17α-hydroxylase, as adrenocortical cells senesce in culture. Proc. Natl. Acad. Sci. USA 84, 1580–1584 (1987).
Ryan, R.F., Hancock, J.P., McDonald, J. & Hornsby, P.J. Cellular senescence involves stochastic processes causing loss of expression of differentiated function genes: Visualization by in situ hybridization for steroid 17α-hydroxylase in bovine adrenocortical cells. Exp. Cell Res. 180, 36–48 (1989).
Cheng, C.Y. & Hornsby, P.J. Expression of 11 β-hydroxylase and 21-hydroxylase in long-term cultures of bovine adrenocortical cells requires extra cellular matrix factors. Endocrinology 130, 2883–2889 (1992).
Tait, J.F. & Tail, S.A.S. Recent perspectives on the history of the adrenal cortex. [The Sir Henry Dale lecture for 1979].J. Endocrlnol. 83, 3P–24P (1979).
Perkins, L.M. & Payne, A.H. Quantification of P450sccp P45017ap and iron sulfur protein reductase in Leydig cells and adrenals of inbred strains of mice. Endocrinology 123, 2675–2682 (1988).
van Weerden W. M., Bierings H. G., van Steenbrugge G. J., de Jong F. H. & Schroder F. H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 50, 857–861 (1992).
Keeney, D.S., Jenkins, C.M. & Waterman, M.R. Developmentally regulated expression of adrenal 17α-hydroxylase cytochrome P450 in the mouse embryo. Endocrinology 136, 4872–1879 (1995).
Pauly, J.E. Morphological observations on the adrenal cortex of the laboratory rat. Endocrinology 60, 247–264 (1957).
Neville, A.M. & O'Hare, M.J. The Human Adrenal Cortex: Pathology and Biology — An Integrated Approach. (Springer-Verlag, Berlin, 1982).
Friend, D.S. & Gilula, N.B. A distinctive cell contact in the rat adrenal cortex. J. Cell Biol. 53, 148–163 (1972).
Reaven, E., Spicher, M. . & Azhar S. Microvillar channels: A unique plasma membrane compartment for concentrating lipoproteins on the surface of rat adrenal cortical cells. J. Lipid Res. 30, 1551–1560 (1989).
Plump, A.S. et al. Apolipoprotein A-I is required for cholesteryl ester accumulation in steroidogenic cells and for normal adrenal steroid production. J. Clin. Invest. 97 2660–2671 (1996).
Idelman, S. The ultrastructure of the mammalian adrenal cortex. Int. Rev. Cytol. 27 181–281 (1970).
Apkarian, R.P. & Curtis, J.C. Hormonal regulation of capillary fenestrae in the rat adrenal cortex: Quantitative studies using objective lens staging scanning electron microscopy. Scand. Electron Microsc. 1986, 1381–1393 (1986).
Hammond, K.D., Torrance, J.M. & DiDomenico, M. Glucocorticoid receptors in murine erythroleukaemic cells. J. Recept. Res. 7, 667–678 (1987).
Bosma, M.J., Phillips, R.A. & Schuler, W. The scid Mouse: Characterization and Potential Uses. (Springer-Verlag, Berlin, 1989).
Volpe, R. et al. New animal models for human autoimmune thyroid disease: Xenografts of human thyroid tissue in severe combined immunodeficient (SCID) and nude mice. Harm. Metab. Res. 25, 623–627 (1993).
Martin, A. et al. Preservation of functioning human thyroid organoids in the scid mouse. 1. System characterization. J. Clin. Endocrinol. Metab. 77 305–310 (1993).
Wright, N. & Alison, M. The Biology of Epithelial Cell Populations, 1. (Clarendon, Oxford, 1984).
Rotten, C.S. Stem Cells. (Academic Press, London, 1997).
Hornsby, P.J. The regulation of adrenocortical function by control of growth and structure. in: Adrenal Cortex.(eds Anderson, D. C. St Winter, J. S. D.) 1–31 (Butterworths, London, 1985).
Boudreau, N., Myers, C. & Bissell, M.J. From laminin to lamin: Regulation of tissue-specific gene expression by the ECM. Trends Cell Biol. 5, 1–4 (1995).
Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357 (1996).
Li, C.H., Adrenocorticotropin 45. Revised amino acid sequences for sheep and bovine hormones. Biochem. Biophys. Res. Common. 49, 835–839 (1972).
Uhler, M., Herbert, E., D'Eustachio, P. & Ruddle, F.D. The mouse genome contains two nonallelic pro-opiomelanocortin genes. J. Biol. Chem. 258, 9444–9453 (1983).
Forough, R. et al. Differential transforming abilities of non-secreted and secreted forms of human fibroblast growth factor-1. J. Biol. Chem. 268, 2960–2968 (1993).
Butcher, R.N., McCullough, K.C., Jarry, C. & Bryant, J., C-treated 3T3/B (3T3/A31) cell feeder layers in hybridoma technology. J. Immunol. Methods 107, 245–251 (1988).
Luo, X., Ikeda, Y. & Parker, K.L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77 481–490 (1994).
Dunn, T.B. Normal and pathologic anatomy of the adrenal gland of the mouse, including neoplasms. J. Notl. Cancer Inst. 44, 1323–1389 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Thomas, M., Northrup, S. & Hornsby, P. Adrenocortical tissue formed by transplantation of normal clones of bovine adrenocortical cells in scid mice replaces the essential functions of the animals' adrenal glands. Nat Med 3, 978–983 (1997). https://doi.org/10.1038/nm0997-978
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0997-978
This article is cited by
-
The adrenal gland microenvironment in health, disease and during regeneration
Hormones (2017)
-
Regulation of the adrenocortical stem cell niche: implications for disease
Nature Reviews Endocrinology (2015)
-
Glucocorticoid signalling affects pancreatic development through both direct and indirect effects
Diabetologia (2006)
-
Efficient restricted gene expression in beta cells by lentivirus-mediated gene transfer into pancreatic stem/progenitor cells
Diabetologia (2005)
-
Cooperation of hTERT, SV40 T Antigen and Oncogenic Ras in Tumorigenesis: A Cell Transplantation Model Using Bovine Adrenocortical Cells
Neoplasia (2002)