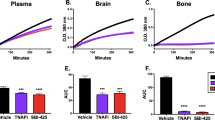
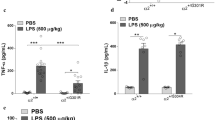
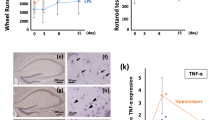
Abstract
The central nervous dysfunctions of lethargy, fever and anorexia are manifestations of sepsis that seem to be mediated by increased cytokine production. Here we demonstrate that tumor necrosis factor (TNF)-α, an essential mediator of endotoxin-induced sepsis, prevents the proteasome-dependent degradation of RGS7, a regulator of G-protein signaling. The stabilization of RGS7 by TNF-α requires activation of the stress-activated protein kinase p38 and the presence of candidate mitogen-activated protein kinase phosphorylation sites. In vivo, RGS7 is rapidly upregulated in mouse brain after exposure to either endotoxin or TNF-α, a response that is nearly abrogated in mice lacking TNF receptor 1. Our findings indicate that TNF-mediated upregulation of RGS7 may contribute to sepsis-induced changes in central nervous function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Haimovitz-Friedman, A. et al. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 186, 1831–1841 (1997).
Selby, P. et al. Tumour necrosis factor in man: clinical and biological observations. Br. J. Cancer 56, 803– 808 (1987).
Waage, A., Brandtzaeg, P., Espevik, T. & Halstensen, A. Current understanding of the pathogenesis of gram-negative shock. Infect. Dis. Clin. North Am. 5, 781– 791 (1991).
Remick, D.G. & Kunkel, S.L. Pathophysiologic alterations induced by tumor necrosis factor. Int. Rev. Exp. Pathol. 34 , 7–25 (1993).
Kapas, L. et al. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am. J. Physiol. 263, R708–715 (1992).
Dunn, A.J. & Swiergiel, A.H. The role of cytokines in infection-related behavior. Ann. NY Acad. Sci. 840, 577– 85 (1998).
Fang, J., Wang, Y. & Krueger, J.M. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J. Neurosci. 17, 5949–5955 (1997).
Dohlman, H.G. & Thorner J. RGS proteins and signaling by heterotrimeric G proteins. J. Biol. Chem. 272, 3871–384 (1997).
Berman, D.M. & Gilman A.G. Mammalian RGS proteins: barbarians at the gate. J. Biol. Chem. 273, 1269–1272 (1998).
Kehrl, J.H. Heterotrimeric G protein signaling: roles in immune function and fine- tuning by RGS proteins. Immunity 8, 1– 10 (1998).
Koelle, M.R. A new family of G-protein regulators - the RGS proteins. Curr. Opin. Cell Biol. 9, 143–147 (1997).
Koelle, M.R., Horvitz HR. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84, 115–125 (1996).
Gold, S.J., Ni, Y.G., Dohlman, H.G. & Nestler, E.J. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J. Neurosci. 17, 8024– 8037 (1997).
Wang, J. et al. RGSZ1, a Gz-selective RGS protein in brain. Structure, membrane association, regulation by galphaz phosphorylation, and relationship to a gz gtpase-activating protein subfamily. J. Biol. Chem. 273, 26014–26025 (1998).
Glick, J.L., Meigs, T.E., Miron, A. & Casey, P.J. RGSZ1, a Gz-selective regulator of G protein signaling whose action is sensitive to the phosphorylation state of gzalpha. J. Biol. Chem. 273, 26008 –26013 (1998).
Ingi, T. et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. J. Neurosci. 18, 7178–7188 (1998).
Shuey, D.J., Betty, M., Jones, P.G., Khawaja, X.Z. & Cockett, M.I. RGS7 attenuates signal transduction through the G(alpha q) family of heterotrimeric G proteins in mammalian cells. J. Neurochem. 70, 1964–1972 ( 1998).
Thomas, E.A., Danielson, P.E. & Sutcliffe, J.G. RGS9: a regulator of G-protein signalling with specific expression in rat and mouse striatum. J. Neurosci. Res. 52, 118–124 (1998).
Saugstad, J.A., Marino, M.J., Folk, J.A., Hepler, J.R. & Conn, P.J. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J. Neurosci. 18, 905– 913 (1998).
Kim, E. et al. Interaction between RGS7 and polycystin. Proc. Natl. Acad. Sci. USA 96, 6371–6376 (1999).
Aberle, H., Bauer, A., Stappert, J., Kispert, A. & Kemler, R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo. J. 16, 3797– 3804 (1997).
Salomon, D. et al. Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system. J. Cell. Biol. 139, 1325–35 (1997).
Abu-Amer, Y., Ross, F.P., Edwards, J. & Teitelbaum, S.L. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its P55 receptor. J. Clin. Invest. 100, 1557– 1565 (1997).
He,W., Cowan, C.W. & Wensel, T.G. RGS9, a GTPase accelerator for phototransduction. Neuron 20, 95–102 (1998).
Khawaja, X.Z. et al. Immunohistochemical distribution of RGS7 protein and cellular selectivity in colocalizing with Galphaq proteins in the adult rat brain. J. Neurochem. 72, 174– 184 (1999).
Segalat, L., Elkes, D.A. & Kaplan, J.M. Modulation of serotonin-controlled behaviors by Go in Caenorhabditis elegans. Science 267, 1648–1651 (1995).
Saitoh, O. et al. RGS7 and RGS8 differentially accelerate G protein-mediated modulation of K+ currents. J. Biol. Chem. 274, 9899–9904 (1999).
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Rechsteiner, M. PEST sequences are signals for rapid intracellular proteolysis. Semin. Cell Biol. 1, 433–440 (1990).
Pulverer, B.J., Kyriakis, J.M., Avruch, J., Nikolakaki, E. & Woodgett, J.R. Phosphorylation of c-jun mediated by MAP kinases. Nature 353, 670– 674 (1991).
Musti, A.M., Treier, M. & Bohmann, D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science 275, 400–402 (1997).
Boone, E., Vandevoorde, V., De Wilde, G. & Haegeman, G. Activation of p42/p44 mitogen-activated protein kinases (MAPK) and p38 MAPK by tumor necrosis factor (TNF) is mediated through the death domain of the 55-kDa TNF receptor. FEBS Lett. 441, 275 –280 (1998).
Arnould, T. et al. The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273 , 6013–6018 (1998).
Acknowledgements
We would like to thank A. Goldberg for advice on this manuscript. T.B. was supported by a fellowship of the Deutsche Forschungsgemeinschaft (DFG). This work was supported by NIH-RO1 DK52897 (G.W.) and PHS grant MH01147 (E.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benzing, T., Brandes, R., Sellin, L. et al. Upregulation of RGS7 may contribute to tumor necrosis factor-induced changes in central nervous function. Nat Med 5, 913–918 (1999). https://doi.org/10.1038/11354
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/11354
This article is cited by
-
RGS7 balances acetylation/de-acetylation of p65 to control chemotherapy-dependent cardiac inflammation
Cellular and Molecular Life Sciences (2023)
-
Arginyltransferase (Ate1) regulates the RGS7 protein level and the sensitivity of light-evoked ON-bipolar responses
Scientific Reports (2021)
-
Clinical Implications of Sarcopenic Obesity in Cancer
Current Oncology Reports (2016)
-
Expression of Neural RGS-R7 and Gβ5 Proteins in Response to Acute and Chronic Morphine
Neuropsychopharmacology (2005)
-
Phosphorylation by casein kinase 2 induces PACS-1 binding of nephrocystin and targeting to cilia
The EMBO Journal (2005)