Abstract
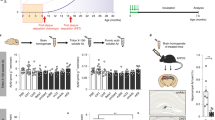
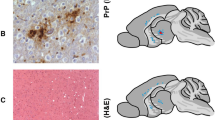
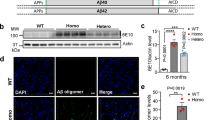
The tissue amyloid deposits that characterize systemic amyloidosis, Alzheimer's disease and the transmissible spongiform encephalopathies always contain serum amyloid P component (SAP) bound to the amyloid fibrils. We have previously proposed that this normal plasma protein may contribute to amyloidogenesis by stabilizing the deposits. Here we show that the induction of reactive amyloidosis is retarded in mice with targeted deletion of the SAP gene. This first demonstration of the participation of SAP in pathogenesis of amyloidosis in vivo confirms that inhibition of SAP binding to amyloid fibrils is an attractive therapeutic target in a range of serious human diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pepys, M.B., Amyloidosis. in Samter's Immunologic Diseases. (eds. Frank, M.M., Austen, K.F., Claman, H.N. & Unanue, E.R.) 637–655 (Little, Brown and Co., Boston, 1994).
Glenner, G.G. Amyloid deposits and amyloidosis — the β-fibrilloses. I. N. Engl. j. Med. 302, 1283–1292 (1980).
Glenner, G.G. Amyloid deposits and amyloidosis — the β-fibrilloses. II. N. Engl. J. Med. 302, 1333–1 343 (1980).
Blake, C.C.F. & Serpell, L.C. Synchrotron X-ray studies suggest that the core of the transthyretin amyloid fibril is a continuous β-sheet helix. Structure 4, 989–998 (1996).
Pepys, M.B. et al. Amyloid P component. A critical review. Amyloid: Int. J. Exp. Clin. Invest. (in the press).
Hawkins, P.N. & Pepys, M.B. Imaging amyloidosis with radiolabelled SAP. Eur. J. Nucl. Med. 22, 595–599 (1995).
Hind, C.R.K., Collins, P.M., Caspi, D., Baltz, M.L. & Pepys, M.B. Specific chemical dissociation of fibrillar and non-fibrillar components of amyloid deposits. Lancet ii, 376–378 (1984).
Pepys, M.B. Amyloid P component: Structure and properties. in Amyloidosis. (eds. Marrink, J. & van Rijswijk, M.H.) 43–50 (Martinus Nijhoff, Dordrecht, the Netherlands, 1986).
Pepys, M.B. et al. Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc. Natl. Acad. Sci. USA 91, 5602–5606 (1994).
Tennent, G.A., Lovat, L.B. & Pepys, M.B. Serum amyloid P component prevents proteolysis of the amyloid fibrils of Alzheimer's disease and systemic amyloidosis. Proc. Natl. Acad. Sci. USA 92, 4299–4303 (1995).
Hawkins, P.N., Tennent, G.A., Woo, P. & Pepys, M.B. Studies in vivo and in vitro of serum amyloid P component in normals and in a patient with AA amyloidosis. Clin. Exp. Immunol. 84, 308–316 (1991).
Kinoshita, C.M. et al. A protease-sensitive site in the proposed Ca2+-binding region of human serum amyloid P component and other pentraxins. Protein Sci. 1, 700–709 (1992).
Hutchinson, W.L., Noble, G.E., Hawkins, P.N. & Pepys, M.B. The pentraxins, C-reactive protein and serum amyloid P component, are cleared and catabolized by hepatocytes in vivo . J. Clin. Invest. 94, 1390–1396 (1994).
Nelson, S.R. et al. Serum amyloid P component in chronic renal failure and dialysis. Clin. Chim. Acta 200, 191–200 (1991).
Cathcart, E.S., Wollheim, F.A. & Cohen, A.S. Immunoassay of P-component in amyloidotic sera. Proc. Soc. Exp. Biol. Med. 125, 1123–1125 (1967).
Hawkins, P.N., Wootton, R. & Pepys, M.B. Metabolic studies of radioiodinated serum amyloid P component in normal subjects and patients with systemic amyloidosis. J. Clin. Invest. 86, 1862–1869 (1990).
Baltz, M.L. et al. Differences in the acute phase responses of serum amyloid P component (SAP) and C3 to injections of casein or bovine serum albumin in amyloid-susceptible and resistant mouse strains. Clin. Exp. Immunol. 39, 355–360 (1980).
Coe, J.E. & Ross, M.J. Amyloidosis and female protein in the Syrian hamster: Concurrent regulation by sex hormones. J. Exp. Med. 171, 1257–1267 (1990).
Snel, F. et al. Experimental amyloidosis in the hamster: Correlation between hamster female protein levels and amyloid deposition. Clin. Exp. Immunol. 76, 296–300 (1989).
Janigan, D.T. Experimental amyloidosis: Studies with a modified casein method, casein hydrolysate and gelatin. Am. J. Pathol. 47, 159–171 (1965).
Benson, M.D., Scheinberg, M.A., Shirahama, T., Cathcart, E.S. & Skinner, M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J. Clin. Invest. 59, 412–417 (1977).
Caspi, D. et al. Imaging of experimental amyloidosis with 131l-serum amyloid P component. Arthritis Rheum. 30, 1303–1306 (1987).
Hawkins, P.N., Myers, M.J., Epenetos, A.A., Caspi, D. & Pepys, M.B. Specific localization and imaging of amyloid deposits in vivo using 123l-labeled serum amyloid P component. J. Exp. Med. 167, 903–913 (1988).
Vigushin, D.M., Pepys, M.B. & Hawkins, P.N. Comparison of histology with SAP scintigraphy for evaluation of amyloidosis. in Amyloid and Amyloidosis 1993. (eds. Kisilevsky, R. et al.) 685–687 (Parthenon Publishing, Pearl River, NY, 1994).
Tan, S.Y., Pepys, M.B. & Hawkins, P.N. Treatment of amyloidosis. Am. J. Kidney Dis. 26, 267–285 (1995).
Kisilevsky, R., Gruys, E. & Shirahama, T. Does amyloid enhancing factor (AEF) exist? Is AEF a single biological entity? Amyloid: Int. J. Exp. Clin. Invest. 2, 128–133 (1995).
Ganowiak, K., Hultman, P., Engstrom, U., Gustavsson, A. & Westermark, P. Fibrils from synthetic amyloid-related peptides enhance development of experimental AA amyloidosis in mice. Biochem. Biophys. Res. Commun. 199, 306–312 (1994).
Booth, D.R. et al. Instability, unfolding and aggregation of human lysozyme variants underlying amyloid fibrillogenesis. Nature 385, 787–793 (1997).
Hawkins, P.N. & Pepys, M.B. A primed state exists in vivo following histological regression of amyloidosis. Clin. Exp. Immunol. 81, 325–328 (1990).
Hawkins, P.N. et al. Serum amyloid P component scintigraphy and turnover studies for diagnosis and quantitative monitoring of AA amyloidosis in juvenile rheumatoid arthritis. Arthritis Rheum. 36, 842–851 (1993).
Coria, F. et al. Isolation and characterization of amyloid P component from Alzheimer's disease and other types of cerebral amyloidosis. Lab. Invest. 58, 454–458 (1988).
Duong, T., Pommier, E.C. & Scheibel, A.B. Immunodetection of the amyloid P component in Alzheimer's disease. Acta Neuropathol. 78, 429–437 (1989).
Kalaria, R.N., Galloway, P.G. & Perry, G. Widespread serum amyloid P immunore-activity in cortical amyloid deposits and the neurofibrillary pathology of Alzheimer's disease and other degenerative disorders. Neuropathol. Appl. Neurobiol. 17, 189–201 (1991).
Kitamoto, T., Tateishi, J., Hikita, K., Nagara, H. & Takeshita, I. A new method to classify amyloid fibril proteins. Acta Neuropathol. 67, 272–278 (1985).
Fricker, J. From mechanisms to drugs in Alzheimer's disease. The Lancet 349, 480 (1997).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Collinge, J. et al. Unaltered susceptibility to BSE in transgenic mice expressing human prion protein. Nature 378, 779–783 (1995).
Axelrad, M.A., Kisilevsky, R., Willmer, J., Chen, S.J. & Skinner, M. Further characterisation of amyloid-enhancing factor. Lab. Invest. 47, 139–146 (1982).
Pepys, M.B., Baltz, M., Comer, K., Davies, A.J.S. & Doenhoff, M. Serum amyloid P-component is an acute-phase reactant in the mouse. Nature 278, 259–261 (1979).
Pepys, M.B. Isolation of serum amyloid P component (protein SAP) in the mouse. Immunology 37, 637–641 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Botto, M., Hawkins, P., Bickerstaff, M. et al. Amyloid deposition is delayed in mice with targeted deletion of the serum amyloid P component gene. Nat Med 3, 855–859 (1997). https://doi.org/10.1038/nm0897-855
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm0897-855
This article is cited by
-
Molecular mechanisms and emerging therapies in wild-type transthyretin amyloid cardiomyopathy
Heart Failure Reviews (2024)
-
Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology
Molecular Neurodegeneration (2021)
-
Serum amyloid P component is an essential element of resistance against Aspergillus fumigatus
Nature Communications (2021)
-
Insights from the in silico structural, functional and phylogenetic characterization of canine lysyl oxidase protein
Journal of Genetic Engineering and Biotechnology (2020)
-
Multifaceted anti-amyloidogenic and pro-amyloidogenic effects of C-reactive protein and serum amyloid P component in vitro
Scientific Reports (2016)